Stereoselectively fluorinated N-heterocycles: a brief survey
- PMID: 24367435
- PMCID: PMC3869242
- DOI: 10.3762/bjoc.9.306
Stereoselectively fluorinated N-heterocycles: a brief survey
Abstract
The stereoselective incorporation of fluorine atoms into N-heterocycles can lead to dramatic changes in the molecules' physical and chemical properties. These changes can be rationally exploited for the benefit of diverse fields such as medicinal chemistry and organocatalysis. This brief review will examine some of the effects that fluorine substitution can have in N-heterocycles, including changes to the molecules' stability, their conformational behaviour, their hydrogen bonding ability, and their basicity. Finally, some methods for the synthesis of stereoselectively fluorinated N-heterocycles will also be reviewed.
Keywords: N-heterocycles; conformation; fluorine; iminosugars; medicinal chemistry; organo-fluorine.
Figures
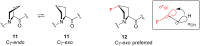
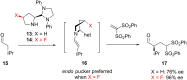

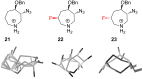

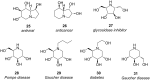






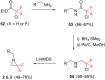
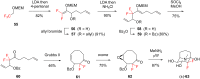
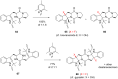
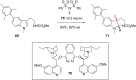


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
