Flow microreactor synthesis in organo-fluorine chemistry
- PMID: 24367443
- PMCID: PMC3869211
- DOI: 10.3762/bjoc.9.314
Flow microreactor synthesis in organo-fluorine chemistry
Abstract
Organo-fluorine compounds are the substances of considerable interest in various industrial fields due to their unique physical and chemical properties. Despite increased demand in wide fields of science, synthesis of fluoro-organic compounds is still often faced with problems such as the difficulties in handling of fluorinating reagents and in controlling of chemical reactions. Recently, flow microreactor synthesis has emerged as a new methodology for producing chemical substances with high efficiency. This review outlines the successful examples of synthesis and reactions of fluorine-containing molecules by the use of flow microreactor systems to overcome long-standing problems in fluorine chemistry.
Keywords: defluorination; flow microreactor; fluorination; fluorine; organo-fluorine; perfluoroalkylation.
Figures
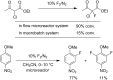
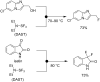
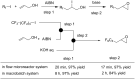
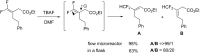
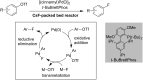
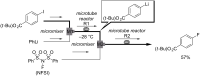
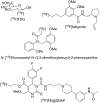
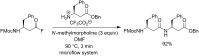
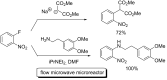
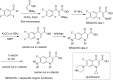
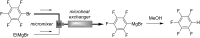
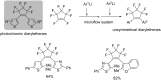
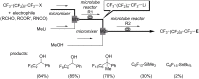
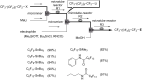
References
-
- Banks R E, Smart B E, Tatlow J C. Organofluorine Chemistry: Principles and Commercial Applications. New York: Plenum Press; 2000.
-
- Hiyama T, Kanie K, Kusumoto T, et al. Organofluorine Compounds: Chemistry and Application. Berlin: Springer-Verlag; 2000. - DOI
-
- Kirsch P. Modern Fluoroorganic Chemistry. Weinheim: Wiley-VCH; 2004. - DOI
-
- Chambers R D. Fluorine in Organic Chemistry. Oxford: Blackwell; 2004. - DOI
-
- Uneyama K. Organofluorine Chemistry. Oxford: Blackwell; 2006. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
