Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada
- PMID: 24367482
- PMCID: PMC3867319
- DOI: 10.1371/journal.pone.0081355
Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada
Abstract
Background: Combination antiretroviral therapy (ART) has significantly increased survival among HIV-positive adults in the United States (U.S.) and Canada, but gains in life expectancy for this region have not been well characterized. We aim to estimate temporal changes in life expectancy among HIV-positive adults on ART from 2000-2007 in the U.S. and Canada.
Methods: Participants were from the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), aged ≥20 years and on ART. Mortality rates were calculated using participants' person-time from January 1, 2000 or ART initiation until death, loss to follow-up, or administrative censoring December 31, 2007. Life expectancy at age 20, defined as the average number of additional years that a person of a specific age will live, provided the current age-specific mortality rates remain constant, was estimated using abridged life tables.
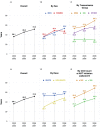
Results: The crude mortality rate was 19.8/1,000 person-years, among 22,937 individuals contributing 82,022 person-years and 1,622 deaths. Life expectancy increased from 36.1 [standard error (SE) 0.5] to 51.4 [SE 0.5] years from 2000-2002 to 2006-2007. Men and women had comparable life expectancies in all periods except the last (2006-2007). Life expectancy was lower for individuals with a history of injection drug use, non-whites, and in patients with baseline CD4 counts <350 cells/mm(3).
Conclusions: A 20-year-old HIV-positive adult on ART in the U.S. or Canada is expected to live into their early 70 s, a life expectancy approaching that of the general population. Differences by sex, race, HIV transmission risk group, and CD4 count remain.
Conflict of interest statement
Figures

References
-
- Boyd MA (2009) Improvements in antiretroviral therapy outcomes over calendar time. Curr Opin HIV AIDS 4: 194–199. - PubMed
-
- Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, et al. (2012) Antiretroviral Treatment of Adult HIV Infection: 2012 Recommendations of the International Antiviral Society–USA Panel. JAMA 308: 387–402. - PubMed
Publication types
MeSH terms
Grants and funding
- U01 AI038855/AI/NIAID NIH HHS/United States
- P30-AI50410/AI/NIAID NIH HHS/United States
- Z01-CP010176/CP/NCI NIH HHS/United States
- R01 AA016893/AA/NIAAA NIH HHS/United States
- U01-AI34989/AI/NIAID NIH HHS/United States
- P30 AI027767/AI/NIAID NIH HHS/United States
- U01 AI035042/AI/NIAID NIH HHS/United States
- U01-AI38855/AI/NIAID NIH HHS/United States
- CBR-86906/CAPMC/ CIHR/Canada
- R01-AA16893/AA/NIAAA NIH HHS/United States
- U01 AI069434/AI/NIAID NIH HHS/United States
- M01 RR000079/RR/NCRR NIH HHS/United States
- R24-AI067039/AI/NIAID NIH HHS/United States
- U01 AI037984/AI/NIAID NIH HHS/United States
- U01-HD32632/HD/NICHD NIH HHS/United States
- R01 DA011602/DA/NIDA NIH HHS/United States
- K23 EY013707/EY/NEI NIH HHS/United States
- K01 AI071725/AI/NIAID NIH HHS/United States
- P30-AI27763/AI/NIAID NIH HHS/United States
- U01-AI34993/AI/NIAID NIH HHS/United States
- K24 AI65298/AI/NIAID NIH HHS/United States
- K23-EY013707/EY/NEI NIH HHS/United States
- U01-AI35043/AI/NIAID NIH HHS/United States
- Z01 CP010176/ImNIH/Intramural NIH HHS/United States
- AI-69432/AI/NIAID NIH HHS/United States
- M01-RR00083/RR/NCRR NIH HHS/United States
- TGF-96118/CAPMC/ CIHR/Canada
- U01 AI031834/AI/NIAID NIH HHS/United States
- HCP-97105/CAPMC/ CIHR/Canada
- U01-AI38858/AI/NIAID NIH HHS/United States
- UM1 AI069434/AI/NIAID NIH HHS/United States
- M01-RR00079/RR/NCRR NIH HHS/United States
- U01 AI035004/AI/NIAID NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- P30 AI054999/AI/NIAID NIH HHS/United States
- R01-DA04334/DA/NIDA NIH HHS/United States
- K24 DA000432/DA/NIDA NIH HHS/United States
- U01 DA036935/DA/NIDA NIH HHS/United States
- U01-AI35040/AI/NIAID NIH HHS/United States
- R01 DA004334/DA/NIDA NIH HHS/United States
- U01-AI37984/AI/NIAID NIH HHS/United States
- M01-RR00071/RR/NCRR NIH HHS/United States
- U10-EY08052/EY/NEI NIH HHS/United States
- U01-AI35004/AI/NIAID NIH HHS/United States
- AI-69434/AI/NIAID NIH HHS/United States
- U10-AA13566/AA/NIAAA NIH HHS/United States
- CDC200-2006-18797/PHS HHS/United States
- U01-AI68636/AI/NIAID NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- U01 AI034989/AI/NIAID NIH HHS/United States
- U01-AI68634/AI/NIAID NIH HHS/United States
- U01 AI037613/AI/NIAID NIH HHS/United States
- KRS-86251/CAPMC/ CIHR/Canada
- M01 RR000071/RR/NCRR NIH HHS/United States
- AHQ290-01-0012/PHS HHS/United States
- U01-AI35042/AI/NIAID NIH HHS/United States
- M01 RR000722/RR/NCRR NIH HHS/United States
- M01-RR025747/RR/NCRR NIH HHS/United States
- CBR-94036/CAPMC/ CIHR/Canada
- U01 AI035041/AI/NIAID NIH HHS/United States
- R24 AI067039/AI/NIAID NIH HHS/United States
- U01-AI37613/AI/NIAID NIH HHS/United States
- M01-RR-00052/RR/NCRR NIH HHS/United States
- U01-AI069918/AI/NIAID NIH HHS/United States
- U01 AI069432/AI/NIAID NIH HHS/United States
- U01-AI42590/AI/NIAID NIH HHS/United States
- U01 AI038858/AI/NIAID NIH HHS/United States
- U10 AA013566/AA/NIAAA NIH HHS/United States
- R01-DA12568/DA/NIDA NIH HHS/United States
- U10 EY008057/EY/NEI NIH HHS/United States
- UL1-RR024131/RR/NCRR NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- U01-AI31834/AI/NIAID NIH HHS/United States
- U01 AI034994/AI/NIAID NIH HHS/United States
- M01 RR000052/RR/NCRR NIH HHS/United States
- K24-DA00432/DA/NIDA NIH HHS/United States
- U10 EY008052/EY/NEI NIH HHS/United States
- P30-AI27757/AI/NIAID NIH HHS/United States
- U01 AA020790/AA/NIAAA NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- K01 AI093197/AI/NIAID NIH HHS/United States
- U01 AI069918/AI/NIAID NIH HHS/United States
- K24 AI065298/AI/NIAID NIH HHS/United States
- U01-AI35041/AI/NIAID NIH HHS/United States
- N02 CP055504/CP/NCI NIH HHS/United States
- U01 AI035043/AI/NIAID NIH HHS/United States
- K23-AI610320/AI/NIAID NIH HHS/United States
- P30 AI027757/AI/NIAID NIH HHS/United States
- UL1 RR025747/RR/NCRR NIH HHS/United States
- U01-AI35039/AI/NIAID NIH HHS/United States
- R01 DA012568/DA/NIDA NIH HHS/United States
- N02-CP55504/CP/NCI NIH HHS/United States
- U10-EY08067/EY/NEI NIH HHS/United States
- P30-AI27767/AI/NIAID NIH HHS/United States
- U01 AI035040/AI/NIAID NIH HHS/United States
- K01-AI093197/AI/NIAID NIH HHS/United States
- U01 AI034993/AI/NIAID NIH HHS/United States
- R01-DA11602/DA/NIDA NIH HHS/United States
- M01 RR000083/RR/NCRR NIH HHS/United States
- K01-AI071725/AI/NIAID NIH HHS/United States
- U01-AI34994/AI/NIAID NIH HHS/United States
- 169621/CAPMC/ CIHR/Canada
- U01 AI035039/AI/NIAID NIH HHS/United States
- 290-01-0012/PHS HHS/United States
- U10-EY08057/EY/NEI NIH HHS/United States
- U10 EY008067/EY/NEI NIH HHS/United States
- M01-RR00722/RR/NCRR NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- U01 HD032632/HD/NICHD NIH HHS/United States
- U01 AI042590/AI/NIAID NIH HHS/United States
- P30-AI54999/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

