Complementary or alternative medicine as possible determinant of decreased persistence to aromatase inhibitor therapy among older women with non-metastatic breast cancer
- PMID: 24367488
- PMCID: PMC3867346
- DOI: 10.1371/journal.pone.0081677
Complementary or alternative medicine as possible determinant of decreased persistence to aromatase inhibitor therapy among older women with non-metastatic breast cancer
Abstract
Purpose: Aromatase inhibitor therapy (AI) significantly improves survival in breast cancer patients. Little is known about adherence and persistence to aromatase inhibitors and about the causes of treatment discontinuation among older women.
Methods: We constituted a cohort of women over 65 receiving a first AI therapy for breast cancer between 2006 and 2008, and followed them until June 2011. Women were selected in the population-based French National Health Insurance databases, and data was collected on the basis of pharmacy refills, medical records and face-to-face interviews. Non-persistence to treatment was defined as the first treatment discontinuation lasting more than 3 consecutive months. Time to treatment discontinuation was studied using survival analysis techniques.
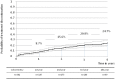
Results: Overall among the 382 selected women, non-persistence to treatment went from 8.7% (95%CI: 6.2-12.1) at 1 year, to 15.6% (95%CI: 12.2-19.8) at 2 years, 20.8% (95%CI: 16.7-25.6) at 3 years, and 24.7% (95%CI: 19.5-31.0) at 4 years. In the multivariate analysis on a sub-sample of 233 women with available data, women using complementary or alternative medicine (CAM) (HR = 3.2; 95%CI: 1.5-6.9) or suffering from comorbidities (HR = 2.2; 95%CI: 1.0-4.8) were more likely to discontinue their treatment, whereas women with polypharmacy (HR = 0.4; 95%CI: 0.2-0.91) were less likely to discontinue. In addition, 13% of the women with positive hormonal receptor status did not fill any prescription for anti-hormonal therapy.
Conclusion: AI therapy is discontinued prematurely in a substantial portion of older patients. Some patients may use CAM not as a complementary treatment, but as an alternative to conventional medicine. Improving patient-physician communication on the use of CAM may improve hormonal therapy adherence.
Conflict of interest statement
Figures
References
-
- Partridge AH, Avorn J, Wang PS, Winer EP (2002) Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst 94: 652–661. - PubMed
-
- Ruddy K, Mayer E, Partridge A (2009) Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin 59: 56–66. - PubMed
-
- Early Breast Cancer Trialists' Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365: 1687–1717. - PubMed
-
- Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, et al. (2004) A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 350: 1081–1092. - PubMed
-
- Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, et al. (2008) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol 9: 45–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical