No evidence of sexual risk compensation in the iPrEx trial of daily oral HIV preexposure prophylaxis
- PMID: 24367497
- PMCID: PMC3867330
- DOI: 10.1371/journal.pone.0081997
No evidence of sexual risk compensation in the iPrEx trial of daily oral HIV preexposure prophylaxis
Abstract
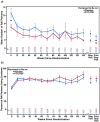
Objective: Preexposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) reduced HIV acquisition in the iPrEx trial among men who have sex with men and transgender women. Self-reported sexual risk behavior decreased overall, but may be affected by reporting bias. We evaluated potential risk compensation using biomarkers of sexual risk behavior.
Design and methods: Sexual practices were assessed at baseline and quarterly thereafter; perceived treatment assignment and PrEP efficacy beliefs were assessed at 12 weeks. Among participants with ≥1 follow-up behavioral assessment, sexual behavior, syphilis, and HIV infection were compared by perceived treatment assignment, actual treatment assignment, and perceived PrEP efficacy.
Results: Overall, acute HIV infection and syphilis decreased during follow-up. Compared with participants believing they were receiving placebo, participants believing they were receiving FTC/TDF reported more receptive anal intercourse partners prior to initiating drug (12.8 vs. 7.7, P = 0.04). Belief in receiving FTC/TDF was not associated with an increase in receptive anal intercourse with no condom (ncRAI) from baseline through follow-up (risk ratio [RR] 0.9, 95% confidence interval [CI]: 0.6-1.4; P = 0.75), nor with a decrease after stopping study drug (RR 0.8, 95% CI: 0.5-1.3; P = 0.46). In the placebo arm, there were trends toward lower HIV incidence among participants believing they were receiving FTC/TDF (incidence rate ratio [IRR] 0.8, 95% CI: 0.4-1.8; P = 0.26) and also believing it was highly effective (IRR 0.5, 95% CI: 0.1-1.7; P = 0.12).
Conclusions: There was no evidence of sexual risk compensation in iPrEx. Participants believing they were receiving FTC/TDF had more partners prior to initiating drug, suggesting that risk behavior was not a consequence of PrEP use.
Conflict of interest statement
Figures
References
-
- UNAIDS (2012) World AIDS Day Report. UNAIDS.
-
- Beyrer C, Baral SD, Walker D, Wirtz AL, Johns B, et al. (2010) The expanding epidemics of HIV type 1 among men who have sex with men in low- and middle-income countries: diversity and consistency. Epidemiol Rev 32: 137–151. - PubMed
-
- van Griensven F, de Lind van Wijngaarden JW, Baral S, Grulich A (2009) The global epidemic of HIV infection among men who have sex with men. Current Opin HIV AIDS 4: 300–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

