Experimental infection of macaques with a wild water bird-derived highly pathogenic avian influenza virus (H5N1)
- PMID: 24367600
- PMCID: PMC3867452
- DOI: 10.1371/journal.pone.0083551
Experimental infection of macaques with a wild water bird-derived highly pathogenic avian influenza virus (H5N1)
Abstract
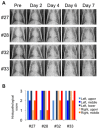
Highly pathogenic avian influenza virus (HPAIV) continues to threaten human health. Non-human primate infection models of human influenza are desired. To establish an animal infection model with more natural transmission and to determine the pathogenicity of HPAIV isolated from a wild water bird in primates, we administered a Japanese isolate of HPAIV (A/whooper swan/Hokkaido/1/2008, H5N1 clade 2.3.2.1) to rhesus and cynomolgus monkeys, in droplet form, via the intratracheal route. Infection of the lower and upper respiratory tracts and viral shedding were observed in both macaques. Inoculation of rhesus monkeys with higher doses of the isolate resulted in stronger clinical symptoms of influenza. Our results demonstrate that HPAIV isolated from a water bird in Japan is pathogenic in monkeys by experimental inoculation, and provide a new method for HPAIV infection of non-human primate hosts, a good animal model for investigation of HPAIV pathogenicity.
Conflict of interest statement
Figures
Similar articles
-
Pathogenesis of bovine H5N1 clade 2.3.4.4b infection in macaques.Nature. 2025 Apr;640(8060):1017-1021. doi: 10.1038/s41586-025-08609-8. Epub 2025 Jan 15. Nature. 2025. PMID: 39814072
-
Distribution of lesions and antigen of highly pathogenic avian influenza virus A/Swan/Germany/R65/06 (H5N1) in domestic cats after presumptive infection by wild birds.Vet Pathol. 2007 May;44(3):261-8. doi: 10.1354/vp.44-3-261. Vet Pathol. 2007. PMID: 17491066
-
Protection against H5N1 highly pathogenic avian and pandemic (H1N1) 2009 influenza virus infection in cynomolgus monkeys by an inactivated H5N1 whole particle vaccine.PLoS One. 2013 Dec 23;8(12):e82740. doi: 10.1371/journal.pone.0082740. eCollection 2013. PLoS One. 2013. PMID: 24376571 Free PMC article.
-
The genetics of highly pathogenic avian influenza viruses of subtype H5 in Germany, 2006-2020.Transbound Emerg Dis. 2021 May;68(3):1136-1150. doi: 10.1111/tbed.13843. Epub 2020 Sep 29. Transbound Emerg Dis. 2021. PMID: 32964686 Review.
-
[Highly pathogenic avian influenza and wild birds].Uirusu. 2009 Jun;59(1):53-8. doi: 10.2222/jsv.59.53. Uirusu. 2009. PMID: 19927989 Review. Japanese.
Cited by
-
Experimental infection of Cynomolgus Macaques with highly pathogenic H5N1 influenza virus through the aerosol route.Sci Rep. 2018 Mar 19;8(1):4801. doi: 10.1038/s41598-018-23022-0. Sci Rep. 2018. PMID: 29556081 Free PMC article.
-
Single Dose of Attenuated Vaccinia Viruses Expressing H5 Hemagglutinin Affords Rapid and Long-Term Protection Against Lethal Infection with Highly Pathogenic Avian Influenza A H5N1 Virus in Mice and Monkeys.Vaccines (Basel). 2025 Jan 15;13(1):74. doi: 10.3390/vaccines13010074. Vaccines (Basel). 2025. PMID: 39852853 Free PMC article.
-
Efficacy of recombinant measles virus expressing highly pathogenic avian influenza virus (HPAIV) antigen against HPAIV infection in monkeys.Sci Rep. 2017 Sep 20;7(1):12017. doi: 10.1038/s41598-017-08326-x. Sci Rep. 2017. PMID: 28931922 Free PMC article.
-
Pathogenesis of bovine H5N1 clade 2.3.4.4b infection in macaques.Nature. 2025 Apr;640(8060):1017-1021. doi: 10.1038/s41586-025-08609-8. Epub 2025 Jan 15. Nature. 2025. PMID: 39814072
-
In Vivo Models to Study the Pathogenesis of Extra-Respiratory Complications of Influenza A Virus Infection.Viruses. 2021 May 6;13(5):848. doi: 10.3390/v13050848. Viruses. 2021. PMID: 34066589 Free PMC article. Review.
References
-
- Chen Y, Deng W, Jia C, Dai X, Zhu H et al. (2009) Pathological lesions and viral localization of influenza A (H5N1) virus in experimentally infected Chinese rhesus macaques: implications for pathogenesis and viral transmission. Arch Virol 154: 227-233. doi:10.1007/s00705-008-0277-5. PubMed: 19130169. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

