Magnetic resonance metabolic profiling of breast cancer tissue obtained with core needle biopsy for predicting pathologic response to neoadjuvant chemotherapy
- PMID: 24367616
- PMCID: PMC3868575
- DOI: 10.1371/journal.pone.0083866
Magnetic resonance metabolic profiling of breast cancer tissue obtained with core needle biopsy for predicting pathologic response to neoadjuvant chemotherapy
Abstract
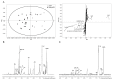
The purpose of this study was to determine whether metabolic profiling of core needle biopsy (CNB) samples using high-resolution magic angle spinning (HR-MAS) magnetic resonance spectroscopy (MRS) could be used for predicting pathologic response to neoadjuvant chemotherapy (NAC) in patients with locally advanced breast cancer. After institutional review board approval and informed consent were obtained, CNB tissue samples were collected from 37 malignant lesions in 37 patients before NAC treatment. The metabolic profiling of CNB samples were performed by HR-MAS MRS. Metabolic profiles were compared according to pathologic response to NAC using the Mann-Whitney test. Multivariate analysis was performed with orthogonal projections to latent structure-discriminant analysis (OPLS-DA). Various metabolites including choline-containing compounds were identified and quantified by HR-MAS MRS in all 37 breast cancer tissue samples obtained by CNB. In univariate analysis, the metabolite concentrations and metabolic ratios of CNB samples obtained with HR-MAS MRS were not significantly different between different pathologic response groups. However, there was a trend of lower levels of phosphocholine/creatine ratio and choline-containing metabolite concentrations in the pathologic complete response group compared to the non-pathologic complete response group. In multivariate analysis, the OPLS-DA models built with HR-MAS MR metabolic profiles showed visible discrimination between the pathologic response groups. This study showed OPLS-DA multivariate analysis using metabolic profiles of pretreatment CNB samples assessed by HR- MAS MRS may be used to predict pathologic response before NAC, although we did not identify the metabolite showing statistical significance in univariate analysis. Therefore, our preliminary results raise the necessity of further study on HR-MAS MR metabolic profiling of CNB samples for a large number of cancers.
Conflict of interest statement
Figures

References
-
- Goldhirsch A, Glick JH, Gelber RD, Coates AS, Senn HJ (2001). Meeting highlights: International Consensus Panel on the Treatment of Primary Breast Cancer. Seventh International Conference on Adjuvant Therapy of Primary Breast Cancer. J Clin Oncol 19: 3817-3827 - PubMed
-
- Abrial SC, Penault-Llorca F, Delva R, Bougnoux P, Leduc B et al. (2005) High prognostic significance of residual disease after neoadjuvant chemotherapy: a retrospective study in 710 patients with operable breast cancer. Breast Cancer Res Treat 94: 255-263. doi:10.1007/s10549-005-9008-8. PubMed: 16267618. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

