Borrelia burgdorferi elicited-IL-10 suppresses the production of inflammatory mediators, phagocytosis, and expression of co-stimulatory receptors by murine macrophages and/or dendritic cells
- PMID: 24367705
- PMCID: PMC3868605
- DOI: 10.1371/journal.pone.0084980
Borrelia burgdorferi elicited-IL-10 suppresses the production of inflammatory mediators, phagocytosis, and expression of co-stimulatory receptors by murine macrophages and/or dendritic cells
Erratum in
- PLoS One. 2014;9(1). doi:10.1371/annotation/2ce59bc4-fcf0-498f-86f0-376432428bf4
- PLoS One. 2014;9(1). doi:10.1371/annotation/680090aa-3e1b-4135-94d6-8082c09180d4
Abstract
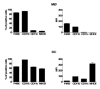
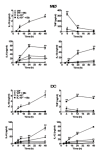
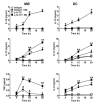
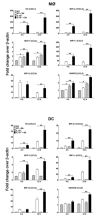
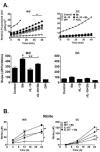
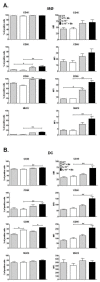
Borrelia burgdorferi (Bb) is a tick-borne spirochete that is the causative agent for Lyme disease. Our previous studies indicate that virulent Bb can potently enhance IL-10 production by macrophages (MØs) and that blocking IL-10 production significantly enhances bacterial clearance. We hypothesize that skin-associated APC types, such as MØs and dendritic cells (DCs) are potent producers of IL-10 in response to Bb, which may act in autocrine fashion to suppress APC responses critical for efficient Bb clearance. Our goal is to delineate which APC immune functions are dysregulated by Bb-elicited IL-10 using a murine model of Lyme disease. Our in vitro studies indicated that both APCs rapidly produce IL-10 upon exposure to Bb, that these levels inversely correlate with the production of many Lyme-relevant proinflammatory cytokines and chemokines, and that APCs derived from IL-10(-/-) mice produced greater amounts of these proinflammatory mediators than wild-type APCs. Phagocytosis assays determined that Bb-elicited IL-10 levels can diminish Bb uptake and trafficking by MØs, suppresses ROS production, but does not affect NO production; Bb-elicited IL-10 had little effect on phagocytosis, ROS, and NO production by DCs. In general, Bb exposure caused little-to-no upregulation of several critical surface co-stimulatory markers by MØs and DCs, however eliminating Bb-elicited IL-10 allowed a significant upregulation in many of these co-stimulatory receptors. These data indicate that IL-10 elicited from Bb-stimulated MØs and DCs results in decreased production of proinflammatory mediators and co-stimulatory molecules, and suppress phagocytosis-associated events that are important for mediating both innate and adaptive immune responses by APCs.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

