Iron, copper, zinc, and manganese transport and regulation in pathogenic Enterobacteria: correlations between strains, site of infection and the relative importance of the different metal transport systems for virulence
- PMID: 24367764
- PMCID: PMC3852070
- DOI: 10.3389/fcimb.2013.00090
Iron, copper, zinc, and manganese transport and regulation in pathogenic Enterobacteria: correlations between strains, site of infection and the relative importance of the different metal transport systems for virulence
Abstract
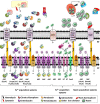
For all microorganisms, acquisition of metal ions is essential for survival in the environment or in their infected host. Metal ions are required in many biological processes as components of metalloproteins and serve as cofactors or structural elements for enzymes. However, it is critical for bacteria to ensure that metal uptake and availability is in accordance with physiological needs, as an imbalance in bacterial metal homeostasis is deleterious. Indeed, host defense strategies against infection either consist of metal starvation by sequestration or toxicity by the highly concentrated release of metals. To overcome these host strategies, bacteria employ a variety of metal uptake and export systems and finely regulate metal homeostasis by numerous transcriptional regulators, allowing them to adapt to changing environmental conditions. As a consequence, iron, zinc, manganese, and copper uptake systems significantly contribute to the virulence of many pathogenic bacteria. However, during the course of our experiments on the role of iron and manganese transporters in extraintestinal Escherichia coli (ExPEC) virulence, we observed that depending on the strain tested, the importance of tested systems in virulence may be different. This could be due to the different set of systems present in these strains, but literature also suggests that as each pathogen must adapt to the particular microenvironment of its site of infection, the role of each acquisition system in virulence can differ from a particular strain to another. In this review, we present the systems involved in metal transport by Enterobacteria and the main regulators responsible for their controlled expression. We also discuss the relative role of these systems depending on the pathogen and the tissues they infect.
Keywords: Enterobacteria; copper; iron; manganese; metal transporters; regulation; virulence; zinc.
Figures

References
-
- Ammendola S., Pasquali P., Pistoia C., Petrucci P., Petrarca P., Rotilio G., et al. (2007). High-affinity Zn2+ uptake system ZnuABC is required for bacterial zinc homeostasis in intracellular environments and contributes to the virulence of Salmonella enterica. Infect. Immun. 75, 5867–5876 10.1128/IAI.00559-07 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

