The use of resazurin as a novel antimicrobial agent against Francisella tularensis
- PMID: 24367766
- PMCID: PMC3853850
- DOI: 10.3389/fcimb.2013.00093
The use of resazurin as a novel antimicrobial agent against Francisella tularensis
Abstract
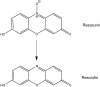
The highly infectious and deadly pathogen, Francisella tularensis, is classified by the CDC as a Category A bioterrorism agent. Inhalation of a single bacterium results in an acute pneumonia with a 30-60% mortality rate without treatment. Due to the prevalence of antibiotic resistance, there is a strong need for new types of antibacterial drugs. Resazurin is commonly used to measure bacterial and eukaryotic cell viability through its reduction to the fluorescent product resorufin. When tested on various bacterial taxa at the recommended concentration of 44 μM, a potent bactericidal effect was observed against various Francisella and Neisseria species, including the human pathogens type A F. tularensis (Schu S4) and N. gonorrhoeae. As low as 4.4 μM resazurin was sufficient for a 10-fold reduction in F. tularensis growth. In broth culture, resazurin was reduced to resorufin by F. tularensis. Resorufin also suppressed the growth of F. tularensis suggesting that this compound is the biologically active form responsible for decreasing the viability of F. tularensis LVS bacteria. Replication of F. tularensis in primary human macrophages and non-phagocytic cells was abolished following treatment with 44 μM resazurin indicating this compound could be an effective therapy for tularemia in vivo.
Keywords: Francisella; Neisseria; antibacterial; antibiotic; macrophages; resazurin; resorufin; tularemia.
Figures
References
-
- Barry E. M., Cole L. E., Santiago A. E. (2009). Vaccines against tularemia. Hum. Vaccin. 5, 832–838 Available online at: https://www.landesbioscience.com/journals/vaccines/article/10297/ or https://www.landesbioscience.com/journals/vaccines/BarryHV5-12.pdf - PMC - PubMed
-
- Bauer J., Siala W., Tulkens P. M., Van Bambeke F. (2013). A combined pharmacodynamic quantitative and qualitative model reveals the potent activity of daptomycin and delafloxacin against Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 57, 2726–2737 10.1128/AAC.00181-13 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

