Tissue-engineered augmentation of a rotator cuff tendon using a reconstituted collagen scaffold: a histological evaluation in sheep
- PMID: 24367785
- PMCID: PMC3838334
Tissue-engineered augmentation of a rotator cuff tendon using a reconstituted collagen scaffold: a histological evaluation in sheep
Abstract
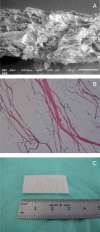
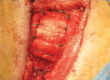
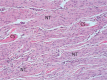
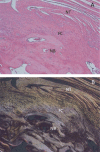
To determine if an absorbable collagen scaffold of high porosity would allow rapid tissue in-growth and permit the functional maturation and alignment of tendon-like tissue, scaffolds were sutured to the superficial surface of the infraspinatus tendons of adult sheep. Histology demonstrated complete ingrowth with fibrovascular tissue by 6 weeks and by 12 weeks the scaffold had induced the formation of a layer of dense, regularly-oriented collagenous tissue which significantly increased the thickness of the native tendon. This new tissue was well-integrated into the host tissues at both the bone interface and along the length of the tendon. At 26 weeks the scaffold was completely absorbed leaving a stable layer of mature tendon-like tissue over the surface of the host tendon which was still present at 52 weeks. The use of a reconstituted collagen scaffold consistently increased the thickness of a rotator cuff tendon by inducing the formation of a well-integrated and mature tendon-like tissue.
Keywords: collagen scaffold; histology; rotator cuff; sheep; tendon.
Figures




Similar articles
-
Rotator Cuff Repair With Autologous Tenocytes and Biodegradable Collagen Scaffold: A Histological and Biomechanical Study in Sheep.Am J Sports Med. 2020 Feb;48(2):450-459. doi: 10.1177/0363546519892580. Epub 2019 Dec 16. Am J Sports Med. 2020. PMID: 31841352
-
A prospective study comparing tendon-to-bone interface healing using an interposition bioresorbable scaffold with a vented anchor for primary rotator cuff repair in sheep.J Shoulder Elbow Surg. 2020 Jan;29(1):157-166. doi: 10.1016/j.jse.2019.05.024. Epub 2019 Aug 7. J Shoulder Elbow Surg. 2020. PMID: 31401128
-
Biologic augmentation of rotator cuff tendon-healing with use of a mixture of osteoinductive growth factors.J Bone Joint Surg Am. 2007 Nov;89(11):2485-97. doi: 10.2106/JBJS.C.01627. J Bone Joint Surg Am. 2007. PMID: 17974893
-
Tissue Engineering for the Ovine Rotator Cuff: Surgical Anatomy, Approach, Implantation and Histology Technique, along with Review of Literature.J Invest Surg. 2020 Feb;33(2):147-158. doi: 10.1080/08941939.2018.1483446. Epub 2018 Oct 19. J Invest Surg. 2020. PMID: 30339484 Review.
-
Strategies in biologic augmentation of rotator cuff repair: a review.Clin Orthop Relat Res. 2010 Jun;468(6):1476-84. doi: 10.1007/s11999-010-1323-7. Clin Orthop Relat Res. 2010. PMID: 20352390 Free PMC article. Review.
Cited by
-
Revision Rotator Cuff Repair With Versus Without an Arthroscopically Inserted Onlay Bioinductive Implant in Workers' Compensation Patients.Orthop J Sports Med. 2023 Jun 6;11(6):23259671231175883. doi: 10.1177/23259671231175883. eCollection 2023 Jun. Orthop J Sports Med. 2023. PMID: 37347026 Free PMC article.
-
Regenerative Engineering of the Rotator Cuff of the Shoulder.ACS Biomater Sci Eng. 2018 Mar 12;4(3):751-786. doi: 10.1021/acsbiomaterials.7b00631. Epub 2018 Feb 6. ACS Biomater Sci Eng. 2018. PMID: 33418763 Free PMC article.
-
Endoscopic Gluteus Medius Repair Augmented With Bioinductive Implant.Arthrosc Tech. 2016 Aug 1;5(4):e821-e825. doi: 10.1016/j.eats.2016.03.011. eCollection 2016 Aug. Arthrosc Tech. 2016. PMID: 27709043 Free PMC article.
-
Retear rates and clinical outcomes at 1 year after repair of full-thickness rotator cuff tears augmented with a bioinductive collagen implant: a prospective multicenter study.JSES Int. 2020 Dec 15;5(2):228-237. doi: 10.1016/j.jseint.2020.10.020. eCollection 2021 Mar. JSES Int. 2020. PMID: 33681842 Free PMC article.
-
Revision arthroscopic surgery after rotator cuff repair with a collagen graft: histologic evaluation of biopsy specimens from two patients.JSES Rev Rep Tech. 2022 Mar 31;2(3):412-418. doi: 10.1016/j.xrrt.2022.02.008. eCollection 2022 Aug. JSES Rev Rep Tech. 2022. PMID: 37588864 Free PMC article. No abstract available.
References
-
- Finnan RP, Crosby LA. Partial-thickness rotator cuff tears. J Shoulder Elbow Surg. 2010;19:609–616. - PubMed
-
- Gartsman GM, Milne JC. Articular surface partial-thickness rotator cuff tears. J Shoulder Elbow Surg. 1995;4:409–415. - PubMed
-
- Sher JS, Uribe JW, Posada A, Murphy BJ, Zlatkin MB. Abnormal findings on magnetic resonance images of asymptomatic shoulders. J Bone Joint Surg Am. 1995;77:10–15. - PubMed
-
- Franceschi F, Papalia R, Del Buono A, Maffulli N, Denaro V. Repair of partial tears of the rotator cuff. Sports Med Arthrosc. 2011;19:401–408. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
