The real-life effectiveness of palivizumab for reducing hospital admissions for respiratory syncytial virus in infants residing in Nunavut
- PMID: 24367792
- PMCID: PMC4128465
- DOI: 10.1155/2014/941367
The real-life effectiveness of palivizumab for reducing hospital admissions for respiratory syncytial virus in infants residing in Nunavut
Abstract
BACKGROUND⁄
Objective: Nunavut has the highest hospitalization rates for respiratory syncytial virus (RSV) worldwide, with rates of 166 per 1000 live births per year <1 year of age. Palivizumab was implemented in Nunavut primarily for premature infants, or those with hemodynamically significant cardiac or chronic lung disease; however, the effectiveness of the program is unknown. The objective of the present multisite, hospital-based surveillance study was to estimate the effectiveness of palivizumab in infants <6 months of age in Nunavut for the 2009 and 2010 RSV seasons.
Methods: Infants identified as palivizumab candidates who were <6 months of age were compared with all admissions for lower respiratory tract infection through multisite, hospital-based surveillance documenting the adequacy of palivizumab prophylaxis, admission for lower respiratory tract infection and the results of RSV testing. The OR for RSV admission in unprophylaxed infants was compared with those who were prophylaxed, and the effectiveness of palivizumab was estimated.
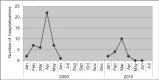
Results: Within the study cohort (n=101) during the two RSV seasons, five of the 10 eligible infants who did not receive adequate prophylaxis were admitted with RSV while two of the 91 infants <6 months of age eligible for palivizumab who were adequately prophylaxed were hospitalized with RSV (OR 22.3 [95% CI 3.8 to 130]; P=0.0005). The estimated effectiveness of palivizumab for the cohort was as high as 96%. Eight eligible infants were missed by the program and did not receive prophylaxis.
Conclusion: Palivizumab was highly effective in reducing hospitalizations due to RSV infection in Nunavut. Further efforts need to be made to ensure that all eligible infants are identified.
HISTORIQUE ET OBJECTIF :: Le Nunavut présente le plus fort taux d’hospitalisations attribuables au virus respiratoire syncytial (VRS) de par le monde, correspondant à 166 cas sur 1 000 naissances vivantes par année chez les moins d’un an. L’utilisation du palivizumaba été implantée au Nunavut, principalement pour les nourrissons prématurés ou ceux qui sont atteints d’une maladie pulmonaire chronique ou cardiaque significative sur le plan hémodynamique. Cependant, on ne connaît pas l’efficacité de ce programme. La présente étude de surveillance multi-hospitalière visait à évaluer l’efficacité du palivizumab chez les nourrissons de moins de six mois au Nunavut pendant les saisons du VRS de 2009 et 2010.
MÉTHODOLOGIE :: Les chercheurs ont comparé les nourrissons qui étaient déterminés comme candidats au palivizumab et qui avaient moins de six mois à toutes les hospitalisations causées par une infection du système respiratoire inférieur par l’entremise d’une surveillance multihospitalière étayant le caractère pertinent de la prophylaxie au palivizumab, des hospitalisations attribuables à une infection du système respiratoire inférieur et des résultats des tests du VRS. Ils ont comparé le rapport de cotes d’hospitalisations attribuables au VRS chez les nourrissons sans prophylaxie à celui des nourrissons sous prophylaxie et évalué l’efficacité du palivizumab.
RÉSULTATS :: Au sein de la cohorte à l’étude (n=101) pendant les deux saisons de VRS, cinq des dix nourrissons admissibles qui n’avaient pas reçu la prophylaxie pertinente ont été hospitalisés en raison d’un VRS tandis que deux des 91 nourrissons de moins de six mois admissibles au palivizumab qui avaient reçu une prophylaxie pertinente ont été hospitalisés pour la même raison (rapport de cotes 22,3 [95 % IC 3,8 à 130]; P=0,0005). L’efficacité estimative du palivizumab dans la cohorte atteignait 96 %. Le programme a omis huit nourris-sons admissibles, qui n’ont pas reçu de prophylaxie.
CONCLUSION :: Le palivizumab réduisait le nombre d’hospitalisations causées par l’infection à VRS avec une grande efficacité au Nunavut. D’autres efforts s’imposent pour s’assurer de dépister tous les nourrissons admissibles.
Figures
Comment in
-
Are we too passive in our attempts to prevent respiratory syncytial virus infection in Northern Canada?Can Respir J. 2014 May-Jun;21(3):163-4. doi: 10.1155/2014/258504. Can Respir J. 2014. PMID: 24914608 Free PMC article. No abstract available.
References
-
- Langley JM, Wang EE, Law BJ, et al. Economic evaluation of respiratory syncytial virus infection in Canadian children: A Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) study. J Pediatr. 1997;131:113–7. - PubMed
-
- Mandell G, Bennett J, Dolin R, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th edn. Philadelphia: Elsevier; 2010.
-
- Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;143(5 Suppl):S127–32. - PubMed
-
- The IMpact-RSV Study Group Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics. 1998;102(3 Pt 1):531–7. - PubMed
-
- Banerji A, Lanctot KL, Paes BA, et al. Comparison of the cost of hospitalization for respiratory syncytial virus disease versus palivizumab prophylaxis in Canadian Inuit infants. Pediatr Infect Dis J. 2009;28:702–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

