Trends in Canadian respiratory clinical trials from 2001 to 2011
- PMID: 24367793
- PMCID: PMC4128464
- DOI: 10.1155/2014/491398
Trends in Canadian respiratory clinical trials from 2001 to 2011
Abstract
Clinical research bridges patients' unmet medical need with innovative medicines, increases knowledge acquisition by clinicians, and creates solutions to improve the sustainability and quality of the Canadian health care system and economy. The Canadian Institutes of Health Research and the Canadian Lung Association have recently raised concerns over declining research activities within the Canadian respiratory community. While there are currently >3000 ongoing clinical trials in Canada, the number of trials investigating common respiratory diseases is unknown. The objective of the present study was to monitor the trends in industry- and non-industry-sponsored respiratory clinical trials in Canada from 2001 to 2011. Trialtrove 2012 (Citeline, an Informa UK business), a database containing summarized clinical trial information regarding pharmaceutical products, was searched using common chronic respiratory disease terms: "allergic rhinitis", "asthma", "chronic obstructive pulmonary disease (COPD)", "cystic fibrosis", "respiratory infections", "pulmonary fibrosis" and "smoking cessation". Over the past 10 years, the number of respiratory clinical trials conducted in Canada has increased (4.49 per year; P=0.004). From 2001 to 2011, the majority of trials were performed in asthma, followed closely by respiratory infections and COPD. Over the past decade, the number of trials investigating COPD and respiratory infections increased (P<0.05), while asthma trials showed a declining trend since 2007. Of the clinical trials performed during this 10-year period, the majority were in phase III, with a significant increase in the number of phase II trials (2.49 per year; P=0.008). However, certain trends observed are concerning and warrant further monitoring in the coming years.
La recherche clinique relie les besoins médicaux non satisfaits des patients aux médecines novatrices, accroît l’acquisition du savoir des cliniciens et crée des solutions pour améliorer la pérennité et la qualité du système et de l’économie de la santé au Canada. Les Instituts de recherche en santé du Canada et l’Association pulmonaire du Canada ont récemment exprimé leur préoccupation à l’égard de la diminution des activités de recherche au sein de la communauté de la santé respiratoire du Canada. Plus de 3 000 essais cliniques sont en cours au Canada, mais on ne sait pas combien portent sur les maladies respiratoires fréquentes. La présente étude visait à surveiller les tendances des essais cliniques en santé respiratoire financés ou non par l’industrie au Canada entre 2001 et 2011. Les chercheurs ont fouillé Trialtrove 2012 (Citeline, une entreprise d’Informa au Royaume-Uni), une base de données contenant de l’information résumée sur les essais cliniques au sujet des produits pharmaceutiques, au moyen de termes courants liés aux maladies respiratoires chroniques : allergic rhinitis, asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis, respiratory infections, pulmonary fibrosis et smoking cessation. Depuis dix ans, le nombre d’essais cliniques en santé respiratoire menés au Canada a augmenté (4,49 par année; P=0,004). De 2001 à 2011, la majorité des essais a porté sur l’asthme, suivie de près par les infections respiratoires et la maladie pulmonaire obstructive chronique (MPOC). Depuis dix ans, le nombre d’essais sur la MPOC et les infections respiratoires a augmenté (P<0,05), tandis que les essais sur l’asthme ont connu une tendance à la baisse depuis 2007. Parmi les essais cliniques effectués pendant cette période de dix ans, la majorité était de phase III, et on constatait une augmentation importante du nombre d’essais de phase II (2,49 par année; P=0,008). Cependant, certaines tendances observées sont inquiétantes et justifient une surveillance supplémentaire au cours des prochaines années.
Figures
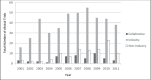


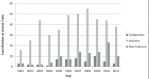
Comment in
-
Respiratory clinical trials in Canada--slow, steady progress toward effective, evidence-based health care.Can Respir J. 2014 May-Jun;21(3):162. doi: 10.1155/2014/296858. Can Respir J. 2014. PMID: 24914607 Free PMC article. No abstract available.
References
-
- Health Canada, CIHR, CLA, Statistics Canada. Respiratory Disease in Canada. < www.phac-aspc.gc.ca/publicat/rdc-mrc01/pdf/rdc0901e.pdf> (Accessed November 13, 2012)
-
- Public Health Agency of Canada. Life and Breath: Respiratory Disease in Canada. < www.phac-aspc.gc.ca/publicat/2007/lbrdcvsmrc/index-eng.php> (Accessed October 22, 2012)
-
- Pharmaceutical Research and Manufacturers of America, Biopharmaceuticals in Perspective, Facts and Figures 2012. Washington, DC: PhRMA; < www.innovation.org/index.cfm/ToolsandResources/Charts#R&D> (Accessed October 23, 2012)
-
- SECOR & KPMG. Improving the Health of Canadians. The contribution of the innovative pharmaceutical industry. < www.canadapharma.org/CMFiles/Our%20Industry/Saving%20Lives%20-%20Transfo...> (Accessed October 22, 2012)
-
- Citeline®, Trialtrove®. Trialtrove® Online Training. < gsk.citeline.com/training/trainings.asp> (Accessed October 3, 2012)
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

