T cell-activation in neuromyelitis optica lesions plays a role in their formation
- PMID: 24367907
- PMCID: PMC3879999
- DOI: 10.1186/2051-5960-1-85
T cell-activation in neuromyelitis optica lesions plays a role in their formation
Abstract
Background: Neuromyelitis optica (NMO) is an inflammatory demyelinating disease of the central nervous system (CNS), which is characterized by the presence of pathogenic serum autoantibodies against aquaporin 4 (AQP4) in the vast majority of patients. The contribution of T cells to the formation of astrocyte destructive lesions is currently unclear. However, active human NMO lesions contain CD4+ T-lymphocytes expressing the activation marker Ox40, and the expression is more profound compared to that seen in MS lesions of comparable activity. Therefore, we analyzed the role of T-cell activation within the CNS in the initiation of NMO lesions in an experimental model of co-transfer of different encephalitogenic T-cells and human AQP4 antibody containing NMO immunoglobulin (NMO IgG). We further studied the expression of the T-cell activation marker Ox40 in NMO and multiple sclerosis lesions in different stages of activity.
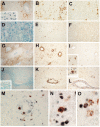
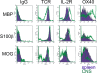
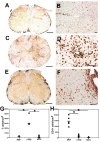
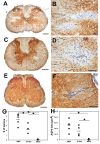
Results: All encephalitogenic T-cell lines used in our experiments induced brain inflammation with a comparable extent of blood brain barrier damage, allowing human NMO IgG to penetrate into the brain and spinal cord tissue. However, astrocyte destructive NMO lesions were only seen with T-cells, which showed signs of activation in the lesions. T-cell activation was reflected by the expression of the activation marker Ox40 and pronounced production of γ-IFN, which was able to increase the production of complement proteins and of the Fc gamma III receptor (Fcgr3) and decreased production of complement inhibitory protein Factor H in microglia.
Conclusions: Our data indicate that local activation of T-cells provide an inflammatory environment in the CNS, which allows AQP4 auto-antibodies to induce astrocyte destructive NMO-like lesions.
Figures



References
-
- Fujihara K, Misu T, Nakashima I. et al.Neuromyelitis optica should be classified as an astrocytopathic disease rather than a demyelinating disease. Clin Exp Neuroimmunol. 2012;1:58–73. doi: 10.1111/j.1759-1961.2012.00030.x. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

