PDGF-responsive progenitors persist in the subventricular zone across the lifespan
- PMID: 24367913
- PMCID: PMC3917572
- DOI: 10.1042/AN20120041
PDGF-responsive progenitors persist in the subventricular zone across the lifespan
Abstract
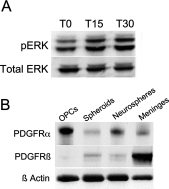
The SVZ (subventricular zone) contains neural stem cells and progenitors of various potentialities. Although initially parsed into A, B, and C cells, this germinal zone is comprised of a significantly more diverse population of cells. Here, we characterized a subset of postnatal PRPs (PDGF-AA-responsive precursors) that express functional PDGFα and β receptors from birth to adulthood. When grown in PDGF-AA, dissociated neonatal rat SVZ cells divided to produce non-adherent clusters of progeny. Unlike the self-renewing EGF/FGF-2-responsive precursors that produce neurospheres, these PRPs failed to self-renew after three passages; therefore, we refer to the colonies they produce as spheroids. Upon differentiation these spheroids could produce neurons, type 1 astrocytes and oligodendrocytes. When maintained in medium supplemented with BMP-4 they also produced type 2 astrocytes. Using lineage tracing methods, it became evident that there were multiple types of PRPs, including a subset that could produce neurons, oligodendrocytes, and type 1 and type 2 astrocytes; thus some of these PRPs represent a unique population of precursors that are quatropotential. Spheroids also could be generated from the newborn neocortex and they had the same potentiality as those from the SVZ. By contrast, the adult neocortex produced less than 20% of the numbers of spheroids than the adult SVZ and spheroids from the adult neocortex only differentiated into glial cells. Interestingly, SVZ spheroid producing capacity diminished only slightly from birth to adulthood. Altogether these data demonstrate that there are PRPs that persist in the SVZ that includes a unique population of quatropotential PRPs.
Figures




Similar articles
-
Multipotential and lineage restricted precursors coexist in the mammalian perinatal subventricular zone.J Neurosci Res. 1997 Apr 15;48(2):83-94. J Neurosci Res. 1997. PMID: 9130137
-
Epidermal growth factor induces the progeny of subventricular zone type B cells to migrate and differentiate into oligodendrocytes.Stem Cells. 2009 Aug;27(8):2032-43. doi: 10.1002/stem.119. Stem Cells. 2009. PMID: 19544429 Free PMC article.
-
mGLU3 metabotropic glutamate receptors modulate the differentiation of SVZ-derived neural stem cells towards the astrocytic lineage.Glia. 2010 May;58(7):813-22. doi: 10.1002/glia.20965. Glia. 2010. PMID: 20091783
-
Oligodendrogenesis in the subventricular zone and the role of epidermal growth factor.Brain Res Rev. 2011 Jun 24;67(1-2):147-56. doi: 10.1016/j.brainresrev.2011.01.001. Epub 2011 Jan 12. Brain Res Rev. 2011. PMID: 21236296 Free PMC article. Review.
-
The Subventricular Zone: A Key Player in Human Neocortical Development.Neuroscientist. 2018 Apr;24(2):156-170. doi: 10.1177/1073858417691009. Epub 2017 Feb 13. Neuroscientist. 2018. PMID: 29254416 Review.
Cited by
-
Mimicking Neural Stem Cell Niche by Biocompatible Substrates.Stem Cells Int. 2016;2016:1513285. doi: 10.1155/2016/1513285. Epub 2016 Jan 6. Stem Cells Int. 2016. PMID: 26880934 Free PMC article. Review.
-
Microglial ASD-related genes are involved in oligodendrocyte differentiation.Sci Rep. 2021 Sep 8;11(1):17825. doi: 10.1038/s41598-021-97257-9. Sci Rep. 2021. PMID: 34497307 Free PMC article.
-
Can Parkinson's disease be cured by stimulating neurogenesis?J Clin Invest. 2015 Mar 2;125(3):978-80. doi: 10.1172/JCI80822. Epub 2015 Feb 17. J Clin Invest. 2015. PMID: 25689259 Free PMC article.
-
Optimizing culture medium composition to improve oligodendrocyte progenitor cell yields in vitro from subventricular zone-derived neural progenitor cell neurospheres.PLoS One. 2015 Apr 2;10(4):e0121774. doi: 10.1371/journal.pone.0121774. eCollection 2015. PLoS One. 2015. PMID: 25837625 Free PMC article.
-
KLF4 Alleviates Hypertrophic Scar Fibrosis by Directly Activating BMP4 Transcription.Int J Biol Sci. 2022 May 9;18(8):3324-3336. doi: 10.7150/ijbs.71167. eCollection 2022. Int J Biol Sci. 2022. PMID: 35637963 Free PMC article.
References
-
- Andrae J, Hansson I, Afink GB, Nister M. Platelet-derived growth factor receptor-alpha in ventricular zone cells and in developing neurons. Mol Cell Neurosci. 2001;17:1001–1013. - PubMed
-
- Assanah MC, Bruce JN, Suzuki SO, Chen A, Goldman JE, Canoll P. PDGF stimulates the massive expansion of glial progenitors in the neonatal forebrain. Glia. 2009;57:1835–1847. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
