Chloroquine synergizes with FTS to enhance cell growth inhibition and cell death
- PMID: 24368422
- PMCID: PMC3960199
- DOI: 10.18632/oncotarget.1500
Chloroquine synergizes with FTS to enhance cell growth inhibition and cell death
Abstract
The Ras family of small GTPases transmits extracellular signals that regulate cell growth, differentiation, motility and death. Ras signaling is constitutively active in a large number of human cancers. Ras can also regulate autophagy by affecting several signaling pathways including the mTOR pathway. Autophagy is a process that regulates the balance between protein synthesis and protein degradation. It is important for normal growth control, but may be defective in diseases. Previously, we have shown that Ras inhibition by FTS induces autophagy, which partially protects cancer cells and may limit the use of FTS as an anti-cancer drug. Since FTS is a non toxic drug we hypothesized that FTS and chloroquine (an autophagy inhibitor) will synergize in cell growth inhibition and cell death. Thus, in the present study, we explored the mechanism of each individual drug and their combined action. Our results demonstrate that in HCT-116 and in Panc-1 cells, FTS induces autophagy, which can be inhibited by chloroquine. Furthermore, the combined treatment synergistically decreased the number of viable cells. Interestingly, the combined treatment enhanced apoptotic cell death as indicated by increased sub-G1 cell population, increased Hoechst staining, activation of caspase 3, decrease in survivin expression and release of cytochrome c. Thus, chloroquine treatment may promote FTS-mediated inhibition of tumor cell growth and may stimulate apoptotic cell death.
Figures
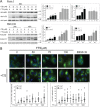


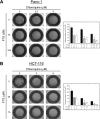
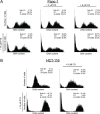


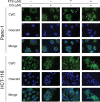
References
-
- Vojtek AB, Der CJ. Increasing complexity of the Ras signaling pathway. The Journal of biological chemistry. 1998;273(32):19925–19928. - PubMed
-
- Downward J. Ras signalling and apoptosis. Current opinion in genetics & development. 1998;8(1):49–54. - PubMed
-
- Harris AL, Nicholson S, Sainsbury R, Wright C, Farndon J. Epidermal growth factor receptor and other oncogenes as prognostic markers. J Natl Cancer Inst Monogr. 1992;(11):181–187. - PubMed
-
- Reddy EP, Reynolds RK, Santos E, Barbacid M. A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene. Nature. 1982;300(5888):149–152. - PubMed
-
- Tabin CJ, Bradley SM, Bargmann CI, Weinberg RA, Papageorge AG, Scolnick EM, Dhar R, Lowy DR, Chang EH. Mechanism of activation of a human oncogene. Nature. 1982;300(5888):143–149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

