The dynamics of diverse segmental amplifications in populations of Saccharomyces cerevisiae adapting to strong selection
- PMID: 24368781
- PMCID: PMC3962480
- DOI: 10.1534/g3.113.009365
The dynamics of diverse segmental amplifications in populations of Saccharomyces cerevisiae adapting to strong selection
Abstract
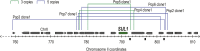
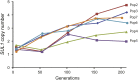
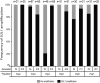
Population adaptation to strong selection can occur through the sequential or parallel accumulation of competing beneficial mutations. The dynamics, diversity, and rate of fixation of beneficial mutations within and between populations are still poorly understood. To study how the mutational landscape varies across populations during adaptation, we performed experimental evolution on seven parallel populations of Saccharomyces cerevisiae continuously cultured in limiting sulfate medium. By combining quantitative polymerase chain reaction, array comparative genomic hybridization, restriction digestion and contour-clamped homogeneous electric field gel electrophoresis, and whole-genome sequencing, we followed the trajectory of evolution to determine the identity and fate of beneficial mutations. During a period of 200 generations, the yeast populations displayed parallel evolutionary dynamics that were driven by the coexistence of independent beneficial mutations. Selective amplifications rapidly evolved under this selection pressure, in particular common inverted amplifications containing the sulfate transporter gene SUL1. Compared with single clones, detailed analysis of the populations uncovers a greater complexity whereby multiple subpopulations arise and compete despite a strong selection. The most common evolutionary adaptation to strong selection in these populations grown in sulfate limitation is determined by clonal interference, with adaptive variants both persisting and replacing one another.
Keywords: clonal interference; evolutionary genomics; experimental evolution; gene amplification; inverted triplication; whole genome sequencing.
Figures



References
-
- Adamo G. M., Lotti M., Tamás M. J., Brocca S., 2012. Amplification of the CUP1 gene is associated with evolution of copper tolerance in Saccharomyces cerevisiae. Microbiology 158: 2325–2335 - PubMed
-
- Andersson D. I., Slechta E. S., Roth J. R., 1998. Evidence that gene amplification underlies adaptive mutability of the bacterial lac operon. Science 282: 1133–1135 - PubMed
-
- Barrick J. E., Yu D. S., Yoon S. H., Jeong H., Oh T. K., et al. , 2009. Genome evolution and adaptation in a long-term experiment with Escherichia coli. Nature 461: 1243–1247 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
