CLASP2 interacts with p120-catenin and governs microtubule dynamics at adherens junctions
- PMID: 24368809
- PMCID: PMC3871427
- DOI: 10.1083/jcb.201306019
CLASP2 interacts with p120-catenin and governs microtubule dynamics at adherens junctions
Abstract
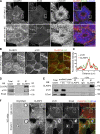
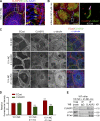
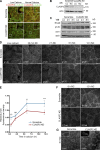
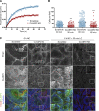
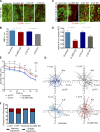
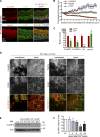
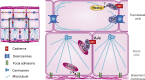
Classical cadherins and their connections with microtubules (MTs) are emerging as important determinants of cell adhesion. However, the functional relevance of such interactions and the molecular players that contribute to tissue architecture are still emerging. In this paper, we report that the MT plus end-binding protein CLASP2 localizes to adherens junctions (AJs) via direct interaction with p120-catenin (p120) in primary basal mouse keratinocytes. Reductions in the levels of p120 or CLASP2 decreased the localization of the other protein to cell-cell contacts and altered AJ dynamics and stability. These features were accompanied by decreased MT density and altered MT dynamics at intercellular junction sites. Interestingly, CLASP2 was enriched at the cortex of basal progenitor keratinocytes, in close localization to p120. Our findings suggest the existence of a new mechanism of MT targeting to AJs with potential functional implications in the maintenance of proper cell-cell adhesion in epidermal stem cells.
Figures


Comment in
-
Microtubules CLASP to Adherens Junctions in epidermal progenitor cells.Bioarchitecture. 2014 Jan-Feb;4(1):25-30. doi: 10.4161/bioa.28177. Epub 2014 Feb 12. Bioarchitecture. 2014. PMID: 24522006 Free PMC article.
References
-
- Akhmanova A., Hoogenraad C.C., Drabek K., Stepanova T., Dortland B., Verkerk T., Vermeulen W., Burgering B.M., De Zeeuw C.I., Grosveld F., Galjart N. 2001. Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell. 104:923–935 10.1016/S0092-8674(01)00288-4 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

