Budget impact analysis of the fentanyl buccal tablet for treatment of breakthrough cancer pain
- PMID: 24368889
- PMCID: PMC3869826
- DOI: 10.2147/CEOR.S52273
Budget impact analysis of the fentanyl buccal tablet for treatment of breakthrough cancer pain
Abstract
Background: The purpose of this study was to assess the economic impact of the fentanyl buccal tablet for the management of breakthrough cancer pain (BTcP) in Spain.
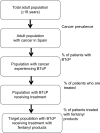
Methods: A 4-year budget impact model was developed for the period 2012-2015 for patients with BTcP from the perspective of the Spanish National Health System. BTcP products included in this model were rapid-onset opioids containing fentanyl (buccal, sublingual, or nasal transmucosal). Prevalence data on cancer, BTcP, opioid use, and number of BTcP episodes were obtained from the literature. Input data on health care resources associated with opioid use and opioid-induced side effects were obtained by consulting experts in oncology from different Spanish hospitals. Resources used included drugs, medical and emergency visits, other nonpharmacologic treatments, and treatment of opioid-induced side effects. Unit costs were obtained from the literature, and a 3% discount rate was applied to costs. Based on the unit costs for drugs and health care resources, the annual BTcP treatment costs per patient associated with each fentanyl product were determined to estimate the overall budget impact based on the total treatment population and the percentage of drug utilization associated with each product. One-way sensitivity analyses were conducted to test the robustness of the model.
Results: Patients treated with oral opioids for BTcP were estimated at 23,291 in 2012, with an increase up to 23,413 in 2015. The average annual budget savings, with an increase of fentanyl buccal tablets, fentanyl sublingual tablets, and intranasal fentanyl spray, and a decrease in oral transmucosal fentanyl citrate, was estimated at €2.6 million, which represents a 0.5% decrease in the total costs of BTcP over the next 4 years. Results of the sensitivity analysis showed that the model was most sensitive to drug cost per day for the fentanyl buccal tablet. A 50% decrease in the daily cost of the fentanyl buccal tablet resulted in the largest overall decrease in budget impact of €5.4 million.
Conclusion: The increase in use of the fentanyl buccal tablet leads to overall savings in the budget impact for the Spanish National Health System. Although the economic impact of treatment for BTcP was shown to increase over 4 years due to population growth, the average annual cost per patient was reduced by €29 with increased use of the fentanyl buccal tablet.
Keywords: breakthrough pain; cancer; costs; economic analysis; rapid onset opioids.
Figures
Similar articles
-
Cost-effectiveness analysis of oral fentanyl formulations for breakthrough cancer pain treatment.PLoS One. 2017 Jun 27;12(6):e0179523. doi: 10.1371/journal.pone.0179523. eCollection 2017. PLoS One. 2017. PMID: 28654672 Free PMC article.
-
An economic evaluation of short-acting opioids for treatment of breakthrough pain in patients with cancer.Value Health. 2011 Mar-Apr;14(2):274-81. doi: 10.1016/j.jval.2010.09.007. Value Health. 2011. PMID: 21402296
-
Single-dose fentanyl sublingual spray for breakthrough cancer pain.Clin Pharmacol. 2013 Jul 24;5:131-41. doi: 10.2147/CPAA.S26649. Print 2013. Clin Pharmacol. 2013. PMID: 23901300 Free PMC article.
-
Efficacy of rapid-onset oral fentanyl formulations vs. oral morphine for cancer-related breakthrough pain: a meta-analysis of comparative trials.J Pain Symptom Manage. 2013 Oct;46(4):573-80. doi: 10.1016/j.jpainsymman.2012.09.009. Epub 2013 Feb 4. J Pain Symptom Manage. 2013. PMID: 23380337 Review.
-
Fentanyl for the treatment of tumor-related breakthrough pain.Dtsch Arztebl Int. 2013 Apr;110(16):271-7. doi: 10.3238/arztebl.2013.0271. Epub 2013 Apr 19. Dtsch Arztebl Int. 2013. PMID: 23671467 Free PMC article. Review.
Cited by
-
Cost-effectiveness analysis of oral fentanyl formulations for breakthrough cancer pain treatment.PLoS One. 2017 Jun 27;12(6):e0179523. doi: 10.1371/journal.pone.0179523. eCollection 2017. PLoS One. 2017. PMID: 28654672 Free PMC article.
-
Breakthrough cancer pain and rational drug use.Support Care Cancer. 2017 Apr;25(Suppl 1):11-17. doi: 10.1007/s00520-017-3636-5. Epub 2017 Feb 18. Support Care Cancer. 2017. PMID: 28213817 Free PMC article.
References
-
- Mercadante S. Cancer pain. Curr Opin Support Palliat Care. 2013;7:139–143. - PubMed
-
- Ventafridda V, Tamburini M, Caraceni A, De Conno F, Naldi F. A validation study of the WHO method for cancer pain relief. Cancer. 1987;59:850–856. - PubMed
-
- Davies AN, Dickman A, Reid C, Stevens AM, Zeppetella G. Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. The management of cancer-related breakthrough pain: recommendations of a task group of the Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. Eur J Pain. 2009;13:331–338. - PubMed
-
- Mercadante S, Portenoy RK. Opioid poorly-responsive cancer pain. Part 1: clinical considerations. J Pain Symptom Manage. 2001;21:144–150. - PubMed
-
- Portenoy RK, Hagen NA. Breakthrough pain: definition, prevalence and characteristics. Pain. 1990;41:273–281. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources