Efficient mitochondrial biogenesis drives incomplete penetrance in Leber's hereditary optic neuropathy
- PMID: 24369379
- PMCID: PMC3914475
- DOI: 10.1093/brain/awt343
Efficient mitochondrial biogenesis drives incomplete penetrance in Leber's hereditary optic neuropathy
Abstract
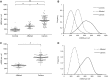
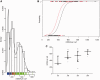
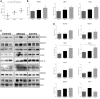
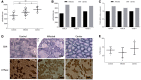
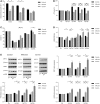
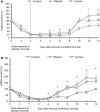
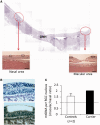
Leber's hereditary optic neuropathy is a maternally inherited blinding disease caused as a result of homoplasmic point mutations in complex I subunit genes of mitochondrial DNA. It is characterized by incomplete penetrance, as only some mutation carriers become affected. Thus, the mitochondrial DNA mutation is necessary but not sufficient to cause optic neuropathy. Environmental triggers and genetic modifying factors have been considered to explain its variable penetrance. We measured the mitochondrial DNA copy number and mitochondrial mass indicators in blood cells from affected and carrier individuals, screening three large pedigrees and 39 independently collected smaller families with Leber's hereditary optic neuropathy, as well as muscle biopsies and cells isolated by laser capturing from post-mortem specimens of retina and optic nerves, the latter being the disease targets. We show that unaffected mutation carriers have a significantly higher mitochondrial DNA copy number and mitochondrial mass compared with their affected relatives and control individuals. Comparative studies of fibroblasts from affected, carriers and controls, under different paradigms of metabolic demand, show that carriers display the highest capacity for activating mitochondrial biogenesis. Therefore we postulate that the increased mitochondrial biogenesis in carriers may overcome some of the pathogenic effect of mitochondrial DNA mutations. Screening of a few selected genetic variants in candidate genes involved in mitochondrial biogenesis failed to reveal any significant association. Our study provides a valuable mechanism to explain variability of penetrance in Leber's hereditary optic neuropathy and clues for high throughput genetic screening to identify the nuclear modifying gene(s), opening an avenue to develop predictive genetic tests on disease risk and therapeutic strategies.
Keywords: LHON penetrance; mitochondrial biogenesis; mtDNA copy number.
Figures


Comment in
-
Weighing in on Leber hereditary optic neuropathy: effects of mitochondrial mass.Brain. 2014 Feb;137(Pt 2):308-9. doi: 10.1093/brain/awu005. Brain. 2014. PMID: 24501072 Free PMC article. No abstract available.
-
Mitochondrial DNA copy number differentiates the Leber's hereditary optic neuropathy affected individuals from the unaffected mutation carriers.Brain. 2016 Jan;139(Pt 1):e1. doi: 10.1093/brain/awv216. Epub 2015 Jul 23. Brain. 2016. PMID: 26209315 No abstract available.
-
Reply: Mitochondrial DNA copy number differentiates the Leber's hereditary optic neuropathy affected individuals from the unaffected mutation carriers.Brain. 2016 Jan;139(Pt 1):e2. doi: 10.1093/brain/awv217. Epub 2015 Jul 23. Brain. 2016. PMID: 26209316 Free PMC article. No abstract available.
References
-
- Baracca A, Solaini G, Sgarbi G, Lenaz G, Baruzzi A, Schapira AH, et al. Severe impairment of complex I-driven adenosine triphosphate synthesis in leber hereditary optic neuropathy cybrids. Arch Neurol. 2005;62:730–6. - PubMed
-
- Barboni P, Carbonelli M, Savini G, Ramos Cdo V, Carta A, Berezovsky A, et al. Natural history of Leber's hereditary optic neuropathy: longitudinal analysis of the retinal nerve fiber layer by optical coherence tomography. Ophthalmology. 2010;117:623–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

