Chromogenic in situ hybridization and p16/Ki67 dual staining on formalin-fixed paraffin-embedded cervical specimens: correlation with HPV-DNA test, E6/E7 mRNA test, and potential clinical applications
- PMID: 24369532
- PMCID: PMC3858005
- DOI: 10.1155/2013/453606
Chromogenic in situ hybridization and p16/Ki67 dual staining on formalin-fixed paraffin-embedded cervical specimens: correlation with HPV-DNA test, E6/E7 mRNA test, and potential clinical applications
Abstract
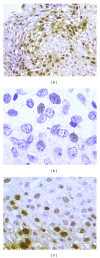
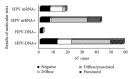
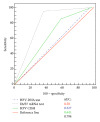
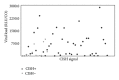
Although HPV-DNA test and E6/E7 mRNA analyses remain the current standard for the confirmation of human papillomavirus (HPV) infections in cytological specimens, no universally adopted techniques exist for the detection of HPV in formalin-fixed paraffin-embedded samples. Particularly, in routine laboratories, molecular assays are still time-consuming and would require a high level of expertise. In this study, we investigated the possible use of a novel HPV tyramide-based chromogenic in situ hybridization (CISH) technology to locate HPV on tissue specimens. Then, we evaluate the potential usefulness of p16(INK4a)/Ki-67 double stain on histological samples, to identify cervical cells expressing HPV E6/E7 oncogenes. In our series, CISH showed a clear signal in 95.2% of the specimens and reached a sensitivity of 86.5%. CISH positivity always matched with HPV-DNA positivity, while 100% of cases with punctated signal joined with cervical intraepithelial neoplasia grade 2 or worse (CIN2+). p16/Ki67 immunohistochemistry gave an interpretable result in 100% of the cases. The use of dual stain significantly increased the agreement between pathologists, which reached 100%. Concordance between dual stain and E6/E7 mRNA test was 89%. In our series, both CISH and p16(INK4a)/Ki67 dual stain demonstrated high grade of performances. In particular, CISH would help to distinguish episomal from integrated HPV, in order to allow conclusions regarding the prognosis of the lesion, while p16(INK4a)/Ki67 dual stain approach would confer a high level of standardization to the diagnostic procedure.
Figures


Similar articles
-
p16/ki67 and E6/E7 mRNA Accuracy and Prognostic Value in Triaging HPV DNA-Positive Women.J Natl Cancer Inst. 2021 Mar 1;113(3):292-300. doi: 10.1093/jnci/djaa105. J Natl Cancer Inst. 2021. PMID: 32745170 Free PMC article.
-
Performance of p16/Ki67 immunostaining, HPV E6/E7 mRNA testing, and HPV DNA assay to detect high-grade cervical dysplasia in women with ASCUS.BMC Cancer. 2019 Mar 27;19(1):271. doi: 10.1186/s12885-019-5492-9. BMC Cancer. 2019. PMID: 30917784 Free PMC article.
-
HR-HPV E6/E7 mRNA In Situ Hybridization: Validation Against PCR, DNA In Situ Hybridization, and p16 Immunohistochemistry in 102 Samples of Cervical, Vulvar, Anal, and Head and Neck Neoplasia.Am J Surg Pathol. 2017 May;41(5):607-615. doi: 10.1097/PAS.0000000000000800. Am J Surg Pathol. 2017. PMID: 28403015
-
HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis.Lancet Oncol. 2014 Nov;15(12):1319-31. doi: 10.1016/S1470-2045(14)70471-1. Epub 2014 Oct 16. Lancet Oncol. 2014. PMID: 25439690
-
Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia.Cancer Epidemiol Biomarkers Prev. 2008 Oct;17(10):2536-45. doi: 10.1158/1055-9965.EPI-08-0306. Cancer Epidemiol Biomarkers Prev. 2008. PMID: 18842994 Free PMC article. Review.
Cited by
-
Cervical intraepithelial neoplasia in pregnancy: Interference of pregnancy status with p16 and Ki-67 protein expression.Oncol Lett. 2017 Jan;13(1):301-306. doi: 10.3892/ol.2016.5441. Epub 2016 Nov 29. Oncol Lett. 2017. PMID: 28123559 Free PMC article.
-
High-Risk HPV CISH Detection in Cervical Biopsies with Weak and/or Focal p16 Immunohistochemical Positivity.Int J Mol Sci. 2024 May 14;25(10):5354. doi: 10.3390/ijms25105354. Int J Mol Sci. 2024. PMID: 38791395 Free PMC article.
-
p16/Ki-67 co-expression associates high risk human papillomavirus persistence and cervical histopathology: a 3-year cohort study in China.Oncotarget. 2016 Oct 4;7(40):64810-64819. doi: 10.18632/oncotarget.11705. Oncotarget. 2016. PMID: 27588487 Free PMC article.
-
Evaluation of Automatic Signal Detection of In Situ Hybridization for Detecting HPV DNA in Cervical Tissue Derived from Patients with Cervical Intraepithelial Neoplasia.Cancers (Basel). 2024 Oct 15;16(20):3485. doi: 10.3390/cancers16203485. Cancers (Basel). 2024. PMID: 39456579 Free PMC article.
-
Sequence variation in the E2-binding domain of HPV16 and biological function evaluation in Tunisian cervical cancers.Biomed Res Int. 2014;2014:639321. doi: 10.1155/2014/639321. Epub 2014 Jun 17. Biomed Res Int. 2014. PMID: 25032221 Free PMC article.
References
-
- Zappacosta R, Rosini S. Cervical cancer screening: from molecular basis to diagnostic practice, going through new technologies. Technology in Cancer Research and Treatment. 2008;7(3):161–174. - PubMed
-
- Cuzick J, Clavel C, Petry K, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. International Journal of Cancer. 2006;119(5):1095–1101. - PubMed
-
- Cuzick J, Arbyn M, Sankaranarayanan R, et al. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008;26(10):K29–K41. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

