Chromogenic in situ hybridization and p16/Ki67 dual staining on formalin-fixed paraffin-embedded cervical specimens: correlation with HPV-DNA test, E6/E7 mRNA test, and potential clinical applications
- PMID: 24369532
- PMCID: PMC3858005
- DOI: 10.1155/2013/453606
Chromogenic in situ hybridization and p16/Ki67 dual staining on formalin-fixed paraffin-embedded cervical specimens: correlation with HPV-DNA test, E6/E7 mRNA test, and potential clinical applications
Abstract
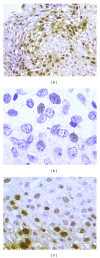
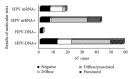
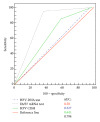
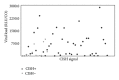
Although HPV-DNA test and E6/E7 mRNA analyses remain the current standard for the confirmation of human papillomavirus (HPV) infections in cytological specimens, no universally adopted techniques exist for the detection of HPV in formalin-fixed paraffin-embedded samples. Particularly, in routine laboratories, molecular assays are still time-consuming and would require a high level of expertise. In this study, we investigated the possible use of a novel HPV tyramide-based chromogenic in situ hybridization (CISH) technology to locate HPV on tissue specimens. Then, we evaluate the potential usefulness of p16(INK4a)/Ki-67 double stain on histological samples, to identify cervical cells expressing HPV E6/E7 oncogenes. In our series, CISH showed a clear signal in 95.2% of the specimens and reached a sensitivity of 86.5%. CISH positivity always matched with HPV-DNA positivity, while 100% of cases with punctated signal joined with cervical intraepithelial neoplasia grade 2 or worse (CIN2+). p16/Ki67 immunohistochemistry gave an interpretable result in 100% of the cases. The use of dual stain significantly increased the agreement between pathologists, which reached 100%. Concordance between dual stain and E6/E7 mRNA test was 89%. In our series, both CISH and p16(INK4a)/Ki67 dual stain demonstrated high grade of performances. In particular, CISH would help to distinguish episomal from integrated HPV, in order to allow conclusions regarding the prognosis of the lesion, while p16(INK4a)/Ki67 dual stain approach would confer a high level of standardization to the diagnostic procedure.
Figures


References
-
- Zappacosta R, Rosini S. Cervical cancer screening: from molecular basis to diagnostic practice, going through new technologies. Technology in Cancer Research and Treatment. 2008;7(3):161–174. - PubMed
-
- Cuzick J, Clavel C, Petry K, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. International Journal of Cancer. 2006;119(5):1095–1101. - PubMed
-
- Cuzick J, Arbyn M, Sankaranarayanan R, et al. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008;26(10):K29–K41. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

