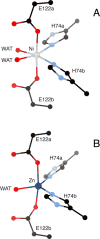
Nonredox nickel enzymes
- PMID: 24369791
- PMCID: PMC5675112
- DOI: 10.1021/cr4004488
Nonredox nickel enzymes
Abstract
Conflict of interest statement
The authors declare no competing financial interest.
Figures

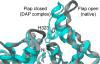
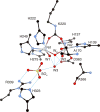
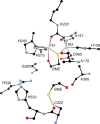
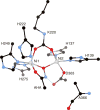
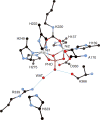
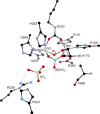
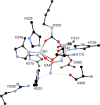
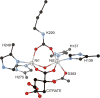



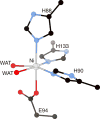
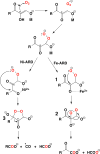
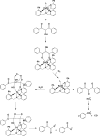
References
-
- Sigel A, Sigel H, Sigel RKO, editors. Nickel and Its Surprising Impact on Nature. John Wiley & Sons, Ltd.; Chichester, England: 2007.
-
- Zambelli B, Ciurli S. Nickel and human health. In: Sigel A, Sigel H, Sigel RKO, editors. Interrelations between Essential Metal Ions and Human Diseases. Vol. 13 Springer Science and Business Media B.V.; Dordrecht, Germany: 2014.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

