Aged male rats regenerate cortical bone with reduced osteocyte density and reduced secretion of nitric oxide after mechanical stimulation
- PMID: 24370615
- PMCID: PMC4791168
- DOI: 10.1007/s00223-013-9832-5
Aged male rats regenerate cortical bone with reduced osteocyte density and reduced secretion of nitric oxide after mechanical stimulation
Abstract
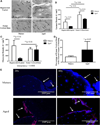
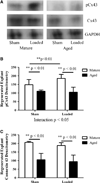
Mechanical loading is integral to the repair of bone damage. Osteocytes are mechanosensors in bone and participate in signaling through gap junction channels, which are primarily comprised of connexin 43 (Cx43). Nitric oxide (NO) and prostaglandin E2 (PGE2) have anabolic and catabolic effects on bone, and the secretion of these molecules occurs after mechanical stimulation. The effect of age on the repair of bone tissue after damage and on the ability of regenerated bone to transduce mechanical stimulation into a cellular response is unexplored. The goal of this study was to examine (1) osteocytes and their mineralized matrix within regenerated bone from aged and mature animals and (2) the ability of regenerated bone explants from aged and mature animals to transduce cyclic mechanical loading into a cellular response through NO and PGE2 secretion. Bilateral cortical defects were created in the diaphysis of aged (21-month-old) or mature (6-month-old) male rats, and new bone tissue was allowed to grow into a custom implant of controlled geometry. Mineralization and mineral-to-matrix ratio were significantly higher in regenerated bone from aged animals, while lacunar and osteocyte density and phosphorylated (pCx43) and total Cx43 protein were significantly lower, relative to mature animals. Regenerated bone from mature rats had increased pCx43 protein and PGE2 secretion with loading and greater NO secretion relative to aged animals. Reduced osteocyte density and Cx43 in regenerated bone in aged animals could limit the establishment of gap junctions as well as NO and PGE2 secretion after loading, thereby altering bone formation and resorption in vivo.
Conflict of interest statement
The authors have stated that they have no conflict of interest.
Figures


Similar articles
-
Osteocyte-Mediated Translation of Mechanical Stimuli to Cellular Signaling and Its Role in Bone and Non-bone-Related Clinical Complications.Curr Osteoporos Rep. 2020 Feb;18(1):67-80. doi: 10.1007/s11914-020-00564-9. Curr Osteoporos Rep. 2020. PMID: 31953640 Review.
-
Cx43 and mechanotransduction in bone.Curr Osteoporos Rep. 2015 Apr;13(2):67-72. doi: 10.1007/s11914-015-0255-2. Curr Osteoporos Rep. 2015. PMID: 25616771 Free PMC article. Review.
-
Effects of mechanical strain on the function of Gap junctions in osteocytes are mediated through the prostaglandin EP2 receptor.J Biol Chem. 2003 Oct 31;278(44):43146-56. doi: 10.1074/jbc.M302993200. Epub 2003 Aug 25. J Biol Chem. 2003. PMID: 12939279
-
Bone marrow stromal cells from aged male rats have delayed mineralization and reduced response to mechanical stimulation through nitric oxide and ERK1/2 signaling during osteogenic differentiation.Biogerontology. 2012 Oct;13(5):467-78. doi: 10.1007/s10522-012-9391-6. Epub 2012 Sep 4. Biogerontology. 2012. PMID: 22944913
-
The Role of Connexin Channels in the Response of Mechanical Loading and Unloading of Bone.Int J Mol Sci. 2020 Feb 9;21(3):1146. doi: 10.3390/ijms21031146. Int J Mol Sci. 2020. PMID: 32050469 Free PMC article. Review.
Cited by
-
Ex vivo Bone Models and Their Potential in Preclinical Evaluation.Curr Osteoporos Rep. 2021 Feb;19(1):75-87. doi: 10.1007/s11914-020-00649-5. Epub 2021 Jan 11. Curr Osteoporos Rep. 2021. PMID: 33428030 Free PMC article. Review.
-
Connexin 43 in the function and homeostasis of osteocytes: a narrative review.Ann Jt. 2023 Dec 19;9:10. doi: 10.21037/aoj-23-65. eCollection 2024. Ann Jt. 2023. PMID: 38529291 Free PMC article. Review.
-
Osteocyte apoptosis: the roles and key molecular mechanisms in resorption-related bone diseases.Cell Death Dis. 2020 Oct 12;11(10):846. doi: 10.1038/s41419-020-03059-8. Cell Death Dis. 2020. PMID: 33046704 Free PMC article. Review.
-
Role of connexins and pannexins during ontogeny, regeneration, and pathologies of bone.BMC Cell Biol. 2016 May 24;17 Suppl 1(Suppl 1):19. doi: 10.1186/s12860-016-0088-6. BMC Cell Biol. 2016. PMID: 27230612 Free PMC article. Review.
-
Senescent and apoptotic osteocytes and aging: Exercise to the rescue?Bone. 2019 Apr;121:255-258. doi: 10.1016/j.bone.2019.02.006. Epub 2019 Feb 6. Bone. 2019. PMID: 30735796 Free PMC article. Review.
References
-
- Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am. 1984;66(3):397–402. - PubMed
-
- Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng. 2006;8:455–498. - PubMed
-
- Szulc P, Garnero P, Munoz F, Marchand F, Delmas PD. Cross-sectional evaluation of bone metabolism in men. J Bone Miner Res. 2001;16(9):1642–1650. - PubMed
-
- Loiselle AE, Jiang JX, Donahue HJ. Gap junction and hemichannel functions in osteocytes. Bone. 2013;54:205–212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

