Inhibition of ataxia telangiectasia mutated (ATM) kinase suppresses herpes simplex virus type 1 (HSV-1) keratitis
- PMID: 24370835
- PMCID: PMC3912898
- DOI: 10.1167/iovs.13-13461
Inhibition of ataxia telangiectasia mutated (ATM) kinase suppresses herpes simplex virus type 1 (HSV-1) keratitis
Abstract
Purpose: Herpes keratitis (HK) remains the leading cause of cornea-derived blindness in the developed world, despite the availability of effective antiviral drugs. Treatment toxicity and the emergence of drug resistance highlight the need for additional therapeutic approaches. This study examined ataxia telangiectasia mutated (ATM), an apical kinase in the host DNA damage response, as a potential new target for the treatment of HK.
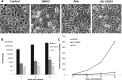
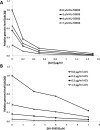
Methods: Small molecule inhibitor of ATM (KU-55933) was used to treat herpes simplex virus type 1 (HSV-1) infection in three experimental models: (1) in vitro--cultured human corneal epithelial cells, hTCEpi, (2) ex vivo--organotypically explanted human and rabbit corneas, and (3) in vivo--corneal infection in young C57BL/6J mice. Infection productivity was assayed by plaque assay, real-time PCR, Western blot, and disease scoring.
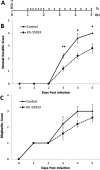
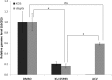
Results: Robust ATM activation was detected in HSV-1-infected human corneal epithelial cells. Inhibition of ATM greatly suppressed viral replication in cultured cells and in explanted human and rabbit corneas, and reduced the severity of stromal keratitis in mice. The antiviral effect of KU-55933 in combination with acyclovir was additive, and KU-55933 suppressed replication of a drug-resistant HSV-1 strain. KU-55933 caused minimal toxicity, as monitored by clonogenic survival assay and fluorescein staining.
Conclusions: This study identifies ATM as a potential target for the treatment of HK. ATM inhibition by KU-55933 reduces epithelial infection and stromal disease severity without producing appreciable toxicity. These findings warrant further investigations into the DNA damage response as an area for therapeutic intervention in herpetic ocular diseases.
Keywords: DNA damage response; HSV-1; ataxia telangiectasia mutated (ATM); cornea; herpes simplex keratitis.
Figures




Similar articles
-
HSV-1 Hijacks the Host DNA Damage Response in Corneal Epithelial Cells through ICP4-Mediated Activation of ATM.Invest Ophthalmol Vis Sci. 2020 Jun 3;61(6):39. doi: 10.1167/iovs.61.6.39. Invest Ophthalmol Vis Sci. 2020. PMID: 32543665 Free PMC article.
-
Targeting host pathways to block HSV-1 at the cornea.Invest Ophthalmol Vis Sci. 2014 Feb 3;55(2):716. doi: 10.1167/iovs.14-13879. Invest Ophthalmol Vis Sci. 2014. PMID: 24492207 No abstract available.
-
Activation of checkpoint kinase 2 is critical for herpes simplex virus type 1 replication in corneal epithelium.Ophthalmic Res. 2015;53(2):55-64. doi: 10.1159/000366228. Epub 2014 Dec 19. Ophthalmic Res. 2015. PMID: 25531207 Free PMC article.
-
[Battle with herpes for 37 years].Nippon Ganka Gakkai Zasshi. 2015 Mar;119(3):145-66; discussion 167. Nippon Ganka Gakkai Zasshi. 2015. PMID: 25854108 Review. Japanese.
-
[Herpes simplex virus latency, reactivation, and a new antiviral therapy for herpetic keratitis].Nippon Ganka Gakkai Zasshi. 2008 Mar;112(3):247-64; discussion 265. Nippon Ganka Gakkai Zasshi. 2008. PMID: 18411713 Review. Japanese.
Cited by
-
HSV-1 Hijacks the Host DNA Damage Response in Corneal Epithelial Cells through ICP4-Mediated Activation of ATM.Invest Ophthalmol Vis Sci. 2020 Jun 3;61(6):39. doi: 10.1167/iovs.61.6.39. Invest Ophthalmol Vis Sci. 2020. PMID: 32543665 Free PMC article.
-
H2AX phosphorylation and DNA damage kinase activity are dispensable for herpes simplex virus replication.Virol J. 2016 Jan 27;13:15. doi: 10.1186/s12985-016-0470-1. Virol J. 2016. PMID: 26817608 Free PMC article.
-
Nuclear antiviral innate responses at the intersection of DNA sensing and DNA repair.Trends Microbiol. 2022 Nov;30(11):1056-1071. doi: 10.1016/j.tim.2022.05.004. Epub 2022 May 28. Trends Microbiol. 2022. PMID: 35641341 Free PMC article. Review.
-
Inflammasome, the Constitutive Heterochromatin Machinery, and Replication of an Oncogenic Herpesvirus.Viruses. 2021 May 6;13(5):846. doi: 10.3390/v13050846. Viruses. 2021. PMID: 34066537 Free PMC article. Review.
-
Blocking HSV-1 glycoprotein K binding to signal peptide peptidase reduces virus infectivity in vitro and in vivo.PLoS Pathog. 2021 Aug 5;17(8):e1009848. doi: 10.1371/journal.ppat.1009848. eCollection 2021 Aug. PLoS Pathog. 2021. PMID: 34352042 Free PMC article.
References
-
- Toma HS, Murina AT, Areaux RG Jr, et al. Ocular HSV-1 latency, reactivation and recurrent disease. Semin Ophthalmol. 2008; 23: 249–273 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

