Human alcohol-related neuropathology
- PMID: 24370929
- PMCID: PMC4532397
- DOI: 10.1007/s00401-013-1233-3
Human alcohol-related neuropathology
Abstract
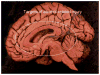
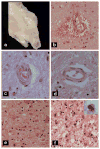
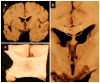
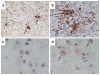
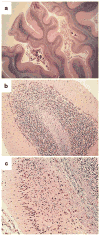
Alcohol-related diseases of the nervous system are caused by excessive exposures to alcohol, with or without co-existing nutritional or vitamin deficiencies. Toxic and metabolic effects of alcohol (ethanol) vary with brain region, age/developmental stage, dose, and duration of exposures. In the mature brain, heavy chronic or binge alcohol exposures can cause severe debilitating diseases of the central and peripheral nervous systems, and skeletal muscle. Most commonly, long-standing heavy alcohol abuse leads to disproportionate loss of cerebral white matter and impairments in executive function. The cerebellum (especially the vermis), cortical-limbic circuits, skeletal muscle, and peripheral nerves are also important targets of chronic alcohol-related metabolic injury and degeneration. Although all cell types within the nervous system are vulnerable to the toxic, metabolic, and degenerative effects of alcohol, astrocytes, oligodendrocytes, and synaptic terminals are major targets, accounting for the white matter atrophy, neural inflammation and toxicity, and impairments in synaptogenesis. Besides chronic degenerative neuropathology, alcoholics are predisposed to develop severe potentially life-threatening acute or subacute symmetrical hemorrhagic injury in the diencephalon and brainstem due to thiamine deficiency, which exerts toxic/metabolic effects on glia, myelin, and the microvasculature. Alcohol also has devastating neurotoxic and teratogenic effects on the developing brain in association with fetal alcohol spectrum disorder/fetal alcohol syndrome. Alcohol impairs function of neurons and glia, disrupting a broad array of functions including neuronal survival, cell migration, and glial cell (astrocytes and oligodendrocytes) differentiation. Further progress is needed to better understand the pathophysiology of this exposure-related constellation of nervous system diseases and better correlate the underlying pathology with in vivo imaging and biochemical lesions.
Figures


Similar articles
-
Interaction of thiamine deficiency and voluntary alcohol consumption disrupts rat corpus callosum ultrastructure.Neuropsychopharmacology. 2007 Oct;32(10):2207-16. doi: 10.1038/sj.npp.1301332. Epub 2007 Feb 14. Neuropsychopharmacology. 2007. PMID: 17299515
-
Cerebral hemodynamic and metabolic effects of chronic alcoholism.Cerebrovasc Brain Metab Rev. 1989 Spring;1(1):2-25. Cerebrovasc Brain Metab Rev. 1989. PMID: 2701368 Review.
-
Development and resolution of brain lesions caused by pyrithiamine- and dietary-induced thiamine deficiency and alcohol exposure in the alcohol-preferring rat: a longitudinal magnetic resonance imaging and spectroscopy study.Neuropsychopharmacology. 2007 May;32(5):1159-77. doi: 10.1038/sj.npp.1301107. Epub 2006 May 24. Neuropsychopharmacology. 2007. PMID: 16723995
-
Alcohol-related amnesia and dementia: animal models have revealed the contributions of different etiological factors on neuropathology, neurochemical dysfunction and cognitive impairment.Neurobiol Learn Mem. 2011 Nov;96(4):596-608. doi: 10.1016/j.nlm.2011.01.003. Epub 2011 Jan 21. Neurobiol Learn Mem. 2011. PMID: 21256970 Free PMC article. Review.
-
Pathophysiology of alcoholic brain damage: synergistic effects of ethanol, thiamine deficiency and alcoholic liver disease.Metab Brain Dis. 1995 Mar;10(1):1-8. doi: 10.1007/BF01991777. Metab Brain Dis. 1995. PMID: 7596324 Review.
Cited by
-
Beneficial effects of octreotide in alcohol-induced neuropathic pain. Role of H 2S, BDNF, TNF-α and Nrf2.Acta Cir Bras. 2021 May 31;36(4):e360408. doi: 10.1590/ACB360408. eCollection 2021. Acta Cir Bras. 2021. PMID: 34076065 Free PMC article.
-
Neuroimaging-Derived Predicted Brain Age and Alcohol Use Among Community-Dwelling Older Adults.Am J Geriatr Psychiatry. 2023 Sep;31(9):669-678. doi: 10.1016/j.jagp.2023.02.043. Epub 2023 Feb 20. Am J Geriatr Psychiatry. 2023. PMID: 36925380 Free PMC article.
-
Accelerated aging and motor control deficits are related to regional deformation of central cerebellar white matter in alcohol use disorder.Addict Biol. 2020 May;25(3):e12746. doi: 10.1111/adb.12746. Epub 2019 Apr 1. Addict Biol. 2020. PMID: 30932270 Free PMC article.
-
Moderate Ethanol-Preconditioning Offers Ischemic Tolerance Against Focal Cerebral Ischemic/Reperfusion: Role of Large Conductance Calcium-Activated Potassium Channel.Neurochem Res. 2022 Dec;47(12):3647-3658. doi: 10.1007/s11064-022-03661-6. Epub 2022 Jul 5. Neurochem Res. 2022. PMID: 35790697
-
Agmatine Production by Aspergillus oryzae Is Elevated by Low pH during Solid-State Cultivation.Appl Environ Microbiol. 2018 Jul 17;84(15):e00722-18. doi: 10.1128/AEM.00722-18. Print 2018 Aug 1. Appl Environ Microbiol. 2018. PMID: 29802188 Free PMC article.
References
-
- Adachi J, Asano M, Ueno Y, et al. Alcoholic muscle disease and biomembrane perturbations (review) J Nutr Biochem. 2003;14(11):616–625. - PubMed
-
- Akai J, Akai K. Neuropathological study of the nucleus basalis of meynert in alcoholic dementia. Arukoru kenkyu to yakubutsu izon = Jpn J Alcohol Stud Drug Depend. 1989;24(2):80–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

