Mature IGF-I excels in promoting functional muscle recovery from disuse atrophy compared with pro-IGF-IA
- PMID: 24371018
- PMCID: PMC3972750
- DOI: 10.1152/japplphysiol.00955.2013
Mature IGF-I excels in promoting functional muscle recovery from disuse atrophy compared with pro-IGF-IA
Abstract
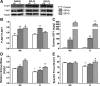
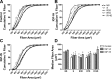
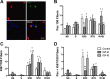
Prolonged disuse of skeletal muscle results in atrophy, and once physical activity is resumed, there is increased susceptibility to injury. Insulin-like growth factor-I (IGF-I) is considered a potential therapeutic target to attenuate atrophy during unloading and to enhance rehabilitation upon reloading of skeletal muscles, due to its multipronged actions on satellite cell proliferation, differentiation, and survival, as well as its actions on muscle fibers to boost protein synthesis and inhibit protein degradation. However, the form of IGF-I delivered may alter the success of treatment. Using the hindlimb suspension model of disuse atrophy, we compared the efficacy of two IGF-I forms in protection against atrophy and enhancement of recovery: mature IGF-I (IGF-IS) lacking the COOH-terminal extension, called the E-peptide, and IGF-IA, which is the predominant form retaining the E-peptide. Self-complementary adeno-associated virus harboring the murine Igf1 cDNA constructs were delivered to hindlimbs of adult female C57BL6 mice 3 days prior to hindlimb suspension. Hindlimb muscles were unloaded for 7 days and then reloaded for 3, 7, and 14 days. Loss of muscle mass following suspension was not prevented by either IGF-I construct. However, IGF-IS expression maintained soleus muscle force production. Further, IGF-IS treatment caused rapid recovery of muscle fiber morphology during reloading and maintained muscle strength. Analysis of gene expression revealed that IGF-IS expression accelerated the downregulation of atrophy-related genes compared with untreated or IGF-IA-treated samples. We conclude that mature-IGF-I may be a better option than pro-IGF-IA to promote skeletal muscle recovery following disuse atrophy.
Keywords: glycosylation; hindlimb suspension; skeletal muscle hypertrophy.
Figures
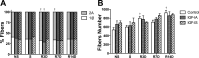

References
-
- Akima H, Ushiyama J, Kubo J, Tonosaki S, Itoh M, Kawakami Y, Fukuoka H, Kanehisa H, Fukunaga T. Resistance training during unweighting maintains muscle size and function in human calf. Med Sci Sports Exerc 35: 655–662, 2003 - PubMed
-
- Allen DL, Roy RR, Edgerton VR. Myonuclear domains in muscle adaptation and disease. Muscle Nerve 22: 1350–1360, 1999 - PubMed
-
- Alzghoul MB, Gerrard D, Watkins BA, Hannon K. Ectopic expression of IGF-I and Shh by skeletal muscle inhibits disuse-mediated skeletal muscle atrophy and bone osteopenia in vivo. FASEB J 18: 221–223, 2004 - PubMed
-
- Barton ER. Impact of sarcoglycan complex on mechanical signal transduction in murine skeletal muscle. Am J Physiol Cell Physiol 290: C411–C419, 2006 - PubMed
-
- Barton ER. The ABCs of IGF-I isoforms: impact on muscle hypertrophy and implications for repair. Appl Physiol Nutr Metab 31: 791–797, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

