Novel attenuated Chikungunya vaccine candidates elicit protective immunity in C57BL/6 mice
- PMID: 24371047
- PMCID: PMC3958085
- DOI: 10.1128/JVI.03453-13
Novel attenuated Chikungunya vaccine candidates elicit protective immunity in C57BL/6 mice
Abstract
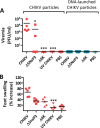
Chikungunya virus (CHIKV) is a reemerging mosquito-borne alphavirus that has caused severe epidemics in Africa and Asia and occasionally in Europe. As of today, there is no licensed vaccine available to prevent CHIKV infection. Here we describe the development and evaluation of novel CHIKV vaccine candidates that were attenuated by deleting a large part of the gene encoding nsP3 or the entire gene encoding 6K and were administered as viral particles or infectious genomes launched by DNA. The resulting attenuated mutants were genetically stable and elicited high magnitudes of binding and neutralizing antibodies as well as strong T cell responses after a single immunization in C57BL/6 mice. Subsequent challenge with a high dose of CHIKV demonstrated that the induced antibody responses protected the animals from viremia and joint swelling. The protective antibody response was long-lived, and a second homologous immunization further enhanced immune responses. In summary, this report demonstrates a straightforward means of constructing stable and efficient attenuated CHIKV vaccine candidates that can be administered either as viral particles or as infectious genomes launched by DNA.
Importance: Similar to other infectious diseases, the best means of preventing CHIKV infection would be by vaccination using an attenuated vaccine platform which preferably raises protective immunity after a single immunization. However, the attenuated CHIKV vaccine candidates developed to date rely on a small number of attenuating point mutations and are at risk of being unstable or even sensitive to reversion. We report here the construction and preclinical evaluation of novel CHIKV vaccine candidates that have been attenuated by introducing large deletions. The resulting mutants proved to be genetically stable, attenuated, highly immunogenic, and able to confer durable immunity after a single immunization. Moreover, these mutants can be administered either as viral particles or as DNA-launched infectious genomes, enabling evaluation of the most feasible vaccine modality for a certain setting. These CHIKV mutants could represent stable and efficient vaccine candidates against CHIKV.
Figures






References
-
- Renault P, Solet JL, Sissoko D, Balleydier E, Larrieu S, Filleul L, Lassalle C, Thiria J, Rachou E, de Valk H, Ilef D, Ledrans M, Quatresous I, Quenel P, Pierre V. 2007. A major epidemic of Chikungunya virus infection on Reunion Island, France, 2005–2006. Am. J. Trop. Med. Hyg. 77:727–731 - PubMed
-
- Schuffenecker I, Iteman I, Michault A, Murri S, Frangeul L, Vaney MC, Lavenir R, Pardigon N, Reynes JM, Pettinelli F, Biscornet L, Diancourt L, Michel S, Duquerroy S, Guigon G, Frenkiel MP, Brehin AC, Cubito N, Despres P, Kunst F, Rey FA, Zeller H, Brisse S. 2006. Genome microevolution of Chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 3:e263. 10.1371/journal.pmed.0030263 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

