Tumor suppressor alterations cooperate to drive aggressive mesotheliomas with enriched cancer stem cells via a p53-miR-34a-c-Met axis
- PMID: 24371224
- PMCID: PMC3945416
- DOI: 10.1158/0008-5472.CAN-13-2062
Tumor suppressor alterations cooperate to drive aggressive mesotheliomas with enriched cancer stem cells via a p53-miR-34a-c-Met axis
Abstract
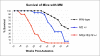
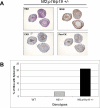
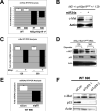
Malignant mesothelioma is a highly aggressive, asbestos-related cancer frequently marked by mutations of both NF2 and CDKN2A. We demonstrate that germline knockout of one allele of each of these genes causes accelerated onset and progression of asbestos-induced malignant mesothelioma compared with asbestos-exposed Nf2(+/-) or wild-type mice. Ascites from some Nf2(+/-);Cdkn2a(+/-) mice exhibited large tumor spheroids, and tail vein injections of malignant mesothelioma cells established from these mice, but not from Nf2(+/-) or wild-type mice, produced numerous tumors in the lung, suggesting increased metastatic potential of tumor cells from Nf2(+/-);Cdkn2a(+/-) mice. Intraperitoneal injections of malignant mesothelioma cells derived from Nf2(+/-);Cdkn2a(+/-) mice into severe combined immunodeficient mice produced tumors that penetrated the diaphragm and pleural cavity and harbored increased cancer stem cells (CSC). Malignant mesothelioma cells from Nf2(+/-);Cdkn2a(+/-) mice stained positively for CSC markers and formed CSC spheroids in vitro more efficiently than counterparts from wild-type mice. Moreover, tumor cells from Nf2(+/-);Cdkn2a(+/-) mice showed elevated c-Met expression/activation, which was partly dependent on p53-mediated regulation of miR-34a and required for tumor migration/invasiveness and maintenance of the CSC population. Collectively, these studies demonstrate in vivo that inactivation of Nf2 and Cdkn2a cooperate to drive the development of highly aggressive malignant mesotheliomas characterized by enhanced tumor spreading capability and the presence of a CSC population associated with p53/miR-34a-dependent activation of c-Met. These findings suggest that cooperativity between losses of Nf2 and Cdkn2a plays a fundamental role in driving the highly aggressive tumorigenic phenotype considered to be a hallmark of malignant mesothelioma.
©2013 AACR.
Figures

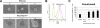

References
-
- Finn RS, Brims FJ, Gandhi A, Olsen N, Musk AW, Maskell NA, et al. Postmortem findings of malignant pleural mesothelioma: a two-center study of 318 patients. Chest. 2012;142:1267–73. - PubMed
-
- Cheng JQ, Jhanwar SC, Klein WM, Bell DW, Lee WC, Altomare DA, et al. p16 alterations and deletion mapping of 9p21-p22 in malignant mesothelioma. Cancer Res. 1994;54:5547–51. - PubMed
-
- Altomare DA, Vaslet CA, Skele KL, De Rienzo A, Devarajan K, Jhanwar SC, et al. A mouse model recapitulating molecular features of human mesothelioma. Cancer Res. 2005;65:8090–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

