A preclinical model of CD38-pretargeted radioimmunotherapy for plasma cell malignancies
- PMID: 24371230
- PMCID: PMC3970848
- DOI: 10.1158/0008-5472.CAN-13-1589
A preclinical model of CD38-pretargeted radioimmunotherapy for plasma cell malignancies
Abstract
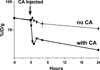
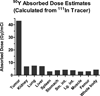
The vast majority of patients with plasma cell neoplasms die of progressive disease despite high response rates to novel agents. Malignant plasma cells are very radiosensitive, but the potential role of radioimmunotherapy (RIT) in the management of plasmacytomas and multiple myeloma has undergone only limited evaluation. Furthermore, CD38 has not been explored as a RIT target despite its uniform high expression on malignant plasma cells. In this report, both conventional RIT (directly radiolabeled antibody) and streptavidin-biotin pretargeted RIT (PRIT) directed against the CD38 antigen were assessed as approaches to deliver radiation doses sufficient for multiple myeloma cell eradication. PRIT demonstrated biodistributions that were markedly superior to conventional RIT. Tumor-to-blood ratios as high as 638:1 were seen 24 hours after PRIT, whereas ratios never exceeded 1:1 with conventional RIT. (90)Yttrium absorbed dose estimates demonstrated excellent target-to-normal organ ratios (6:1 for the kidney, lung, liver; 10:1 for the whole body). Objective remissions were observed within 7 days in 100% of the mice treated with doses ranging from 800 to 1,200 μCi of anti-CD38 pretargeted (90)Y-DOTA-biotin, including 100% complete remissions (no detectable tumor in treated mice compared with tumors that were 2,982% ± 2,834% of initial tumor volume in control animals) by day 23. Furthermore, 100% of animals bearing NCI-H929 multiple myeloma tumor xenografts treated with 800 μCi of anti-CD38 pretargeted (90)Y-DOTA-biotin achieved long-term myeloma-free survival (>70 days) compared with none (0%) of the control animals.
©2013 AACR.
Conflict of interest statement
Disclosure of COI: The authors have no conflicts of interest to disclose.
Figures





References
-
- Pilarski LM, Hipperson G, Seeberger K, Pruski E, Coupland RW, Belch AR. Myeloma progenitors in the blood of patients with aggressive or minimal disease: engraftment and self-renewal of primary human myeloma in the bone marrow of NOD SCID mice. Blood. 2000;95:1056–1065. - PubMed
-
- Nademanee A, Forman S, Molina A, Fung H, Smith D, Dagis A, et al. A phase 1/2 trial of high-dose yttrium-90-ibritumomab tiuxetan in combination with high-dose etoposide and cyclophosphamide followed by autologous stem cell transplantation in patients with poor-risk or relapsed non-Hodgkin lymphoma. Blood. 2005;106:2896–2902. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

