Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy
- PMID: 24371263
- PMCID: PMC3934740
- DOI: 10.1194/jlr.P040501
Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy
Abstract
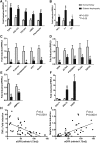
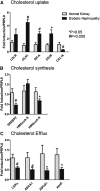
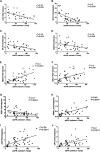
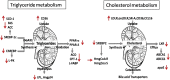
Animal models link ectopic lipid accumulation to renal dysfunction, but whether this process occurs in the human kidney is uncertain. To this end, we investigated whether altered renal TG and cholesterol metabolism results in lipid accumulation in human diabetic nephropathy (DN). Lipid staining and the expression of lipid metabolism genes were studied in kidney biopsies of patients with diagnosed DN (n = 34), and compared with normal kidneys (n = 12). We observed heavy lipid deposition and increased intracellular lipid droplets. Lipid deposition was associated with dysregulation of lipid metabolism genes. Fatty acid β-oxidation pathways including PPAR-α, carnitine palmitoyltransferase 1, acyl-CoA oxidase, and L-FABP were downregulated. Downregulation of renal lipoprotein lipase, which hydrolyzes circulating TGs, was associated with increased expression of angiopoietin-like protein 4. Cholesterol uptake receptor expression, including LDL receptors, oxidized LDL receptors, and acetylated LDL receptors, was significantly increased, while there was downregulation of genes effecting cholesterol efflux, including ABCA1, ABCG1, and apoE. There was a highly significant correlation between glomerular filtration rate, inflammation, and lipid metabolism genes, supporting a possible role of abnormal lipid metabolism in the pathogenesis of DN. These data suggest that renal lipid metabolism may serve as a target for specific therapies aimed at slowing the progression of glomerulosclerosis.
Keywords: cholesterol metabolism; lipid droplets; lipotoxicity.
Figures



References
-
- Rutledge J. C., Ng K. F., Aung H. H., Wilson D. W. 2010. Role of triglyceride-rich lipoproteins in diabetic nephropathy. Nat. Rev. Nephrol. 6: 361–370 - PubMed
-
- Cooper M. E., Jandeleit-Dahm K. A. 2005. Lipids and diabetic renal disease. Curr. Diab. Rep. 5: 445–448 - PubMed
-
- Li W. Y., Yao C. X., Zhang S. F., Wang S. L., Wang T. Q., Xiong C. J., Li Y. B., Zang M. X. 2012. Improvement of myocardial lipid accumulation and prevention of PGC-1α induction by fenofibrate. Mol. Med. Rep. 5: 1396–1400 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

