The long-term immunogenicity of an inactivated split-virion 2009 pandemic influenza A H1N1 vaccine: Randomized, observer-masked, single-center clinical study
- PMID: 24371582
- PMCID: PMC3862378
- DOI: 10.1016/j.rinim.2012.10.001
The long-term immunogenicity of an inactivated split-virion 2009 pandemic influenza A H1N1 vaccine: Randomized, observer-masked, single-center clinical study
Abstract
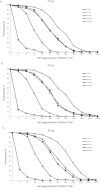
The aim of this study is to investigate the long-term immunogenicity of inactivated split-virion 2009 pandemic influenza A H1N1 vaccine after a single immunization. We recruited 480 adults, aged 18-60 years, for a placebo-controlled, observer-masked, single-center clinical study. We randomly assigned subjects into four groups: 15 μg, 30 μg and 45 μg of hemagglutinin (HA) dosage groups, and a placebo control group. Finally, 259 subjects completed the entire study. The rates of seroconversion and seroprotection and the geometric mean increase (GMI) fulfilled the criteria of the European Medicines Agency (EMEA) for influenza vaccine for 180 days after vaccination in all three dosage groups. However, the seroprotection rates of all dosage groups were below 70% at day 360 post vaccination, while the seroconversion rates and the GMI continued to meet the licensure criteria at this time point. In conclusion, a single dose of 15 μg HA vaccine could induce a protective immune response persisting for at least six months in adults. This study could be beneficial for the future development of influenza vaccines conferring long-term immunity.
Keywords: H1N1; Hemagglutinin (HA); Immunogenicity; Influenza; Long-term.
Figures


References
-
- Smith G.J., Vijaykrishna D., Bahl J., Lycett S.J., Worobey M., Pybus O.G. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122–1125. - PubMed
-
- Pandemic (H1N1) 2009-update 112. WHO, <http://www.who.int/csr/don/2010_08_06/en/index.html>; 2009 [accessed 06.08.10].
-
- Peiris J.S., Tu W.W., Yen H.L. A novel H1N1 virus causes the first pandemic of the 21st century. European Journal ofImmunology. 2009;39:2946–2954. - PubMed
-
- Girard M.P., Tam J.S., Assossou O.M., Kieny M.P. The 2009 A (H1N1) influenza virus pandemic:a review. Vaccine. 2010;28:4895–4902. - PubMed
LinkOut - more resources
Full Text Sources

