μ-Opioid receptor activation and noradrenaline transport inhibition by tapentadol in rat single locus coeruleus neurons
- PMID: 24372103
- PMCID: PMC4292960
- DOI: 10.1111/bph.12566
μ-Opioid receptor activation and noradrenaline transport inhibition by tapentadol in rat single locus coeruleus neurons
Abstract
Background and purpose: Tapentadol is a novel analgesic that combines moderate μ-opioid receptor agonism and noradrenaline reuptake inhibition in a single molecule. Both mechanisms of action are involved in producing analgesia; however, the potency and efficacy of tapentadol in individual neurons has not been characterized.
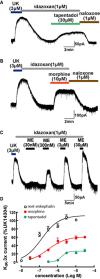
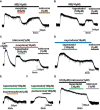
Experimental approach: Whole-cell patch-clamp recordings of G-protein-coupled inwardly rectifying K(+) (KIR 3.x) currents were made from rat locus coeruleus neurons in brain slices to investigate the potency and relative efficacy of tapentadol and compare its intrinsic activity with other clinically used opioids.
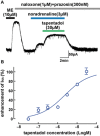
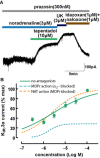
Key results: Tapentadol showed agonist activity at μ receptors and was approximately six times less potent than morphine with respect to KIR 3.x current modulation. The intrinsic activity of tapentadol was lower than [Met]enkephalin, morphine and oxycodone, but higher than buprenorphine and pentazocine. Tapentadol inhibited the noradrenaline transporter (NAT) with potency similar to that at μ receptors. The interaction between these two mechanisms of action was additive in individual LC neurons.
Conclusions and implications: Tapentadol displays similar potency for both µ receptor activation and NAT inhibition in functioning neurons. The intrinsic activity of tapentadol at the μ receptor lies between that of buprenorphine and oxycodone, potentially explaining the favourable profile of side effects, related to μ receptors.
Linked articles: This article is part of a themed section on Opioids: New Pathways to Functional Selectivity. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2015.172.issue-2.
Keywords: GIRK channels; KIR3.x; locus coeruleus; noradrenalin reuptake inhibitor; opioid receptor agonist; tapentadol.
© 2013 The British Pharmacological Society.
Figures
Similar articles
-
Effect of tapentadol on neurons in the locus coeruleus.Neuropharmacology. 2013 Sep;72:250-8. doi: 10.1016/j.neuropharm.2013.04.053. Epub 2013 May 7. Neuropharmacology. 2013. PMID: 23664814
-
Desensitization and Tolerance of Mu Opioid Receptors on Pontine Kölliker-Fuse Neurons.Mol Pharmacol. 2018 Jan;93(1):8-13. doi: 10.1124/mol.117.109603. Epub 2017 Nov 2. Mol Pharmacol. 2018. PMID: 29097440 Free PMC article.
-
The influence of μ-opioid and noradrenaline reuptake inhibition in the modulation of pain responsive neurones in the central amygdala by tapentadol in rats with neuropathy.Eur J Pharmacol. 2015 Feb 15;749:151-60. doi: 10.1016/j.ejphar.2014.11.032. Epub 2015 Jan 6. Eur J Pharmacol. 2015. PMID: 25576174 Free PMC article.
-
The mu-opioid receptor agonist/noradrenaline reuptake inhibition (MOR-NRI) concept in analgesia: the case of tapentadol.CNS Drugs. 2014 Apr;28(4):319-29. doi: 10.1007/s40263-014-0151-9. CNS Drugs. 2014. PMID: 24578192 Review.
-
[Tapentadol: with two mechanisms of action in one molecule effective against nociceptive and neuropathic pain. Preclinical overview].Schmerz. 2011 Feb;25(1):19-25. doi: 10.1007/s00482-010-1004-1. Schmerz. 2011. PMID: 21258822 Review. German.
Cited by
-
A tetrapeptide class of biased analgesics from an Australian fungus targets the µ-opioid receptor.Proc Natl Acad Sci U S A. 2019 Oct 29;116(44):22353-22358. doi: 10.1073/pnas.1908662116. Epub 2019 Oct 14. Proc Natl Acad Sci U S A. 2019. PMID: 31611414 Free PMC article.
-
Themed section.Br J Pharmacol. 2015 Jan;172(2):247-50. doi: 10.1111/bph.13028. Br J Pharmacol. 2015. PMID: 25537825 Free PMC article.
-
Tapentadol Extended Release in the Treatment of Severe Chronic Low Back Pain and Osteoarthritis Pain.Pain Ther. 2018 Jun;7(1):37-57. doi: 10.1007/s40122-018-0095-8. Epub 2018 Apr 5. Pain Ther. 2018. PMID: 29623654 Free PMC article. Review.
-
Tapentadol shows lower intrinsic efficacy at µ receptor than morphine and oxycodone.Pharmacol Res Perspect. 2022 Feb;10(1):e00921. doi: 10.1002/prp2.921. Pharmacol Res Perspect. 2022. PMID: 35084120 Free PMC article.
-
Analysis of Opioid-Related Adverse Events in Japan Using FAERS Database.Pharmaceuticals (Basel). 2023 Nov 1;16(11):1541. doi: 10.3390/ph16111541. Pharmaceuticals (Basel). 2023. PMID: 38004407 Free PMC article.
References
-
- Afilalo M, Etropolski MS, Kuperwasser B, Kelly K, Okamoto A, Van Hove I, et al. Efficacy and safety of Tapentadol extended release compared with oxycodone controlled release for the management of moderate to severe chronic pain related to osteoarthritis of the knee: a randomized, double-blind, placebo- and active-controlled phase III study. Clin Drug Investig. 2010;30:489–505. - PubMed
-
- Bee LA, Bannister K, Rahman W, Dickenson AH. Mu-opioid and noradrenergic alpha(2)-adrenoceptor contributions to the effects of tapentadol on spinal electrophysiological measures of nociception in nerve-injured rats. Pain. 2011;152:131–139. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

