Ultrasound and microbubble guided drug delivery: mechanistic understanding and clinical implications
- PMID: 24372231
- PMCID: PMC4084724
- DOI: 10.2174/1389201014666131226114611
Ultrasound and microbubble guided drug delivery: mechanistic understanding and clinical implications
Abstract
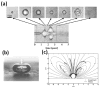
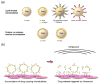
Ultrasound mediated drug delivery using microbubbles is a safe and noninvasive approach for spatially localized drug administration. This approach can create temporary and reversible openings on cellular membranes and vessel walls (a process called "sonoporation"), allowing for enhanced transport of therapeutic agents across these natural barriers. It is generally believed that the sonoporation process is highly associated with the energetic cavitation activities (volumetric expansion, contraction, fragmentation, and collapse) of the microbubble. However, a thorough understanding of the process was unavailable until recently. Important progress on the mechanistic understanding of sonoporation and the corresponding physiological responses in vitro and in vivo has been made. Specifically, recent research shed light on the cavitation process of microbubbles and fluid motion during insonation of ultrasound, on the spatio-temporal interactions between microbubbles and cells or vessel walls, as well as on the temporal course of the subsequent biological effects. These findings have significant clinical implications on the development of optimal treatment strategies for effective drug delivery. In this article, current progress in the mechanistic understanding of ultrasound and microbubble mediated drug delivery and its implications for clinical translation is discussed.
Conflict of interest statement
The authors confirm that this article content has no conflicts of interest.
Figures

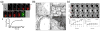
References
-
- Szabo TL. Diagnostic ultrasound imaging: inside out. 2004. Access Online via Elsevier.
-
- Dubinsky TJ, Cuevas C, Dighe MK, Kolokythas O, Joo HH. High-intensity focused ultrasound: Current potential and oncologic applications. Am J Roentgenology. 2008;190(1):191–199. - PubMed
-
- Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5(4):321–327. - PubMed
-
- Roberts WW, Hall TL, Ives K, Wolf JS, Jr, Fowlkes JB, Cain CA. Pulsed cavitational ultrasound: a noninvasive technology for controlled tissue ablation (histotripsy) in the rabbit kidney. J Urol. 2006;175(2):734–738. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
