Prevalence, nature and potential preventability of adverse drug events - a population-based medical record study of 4970 adults
- PMID: 24372506
- PMCID: PMC4168391
- DOI: 10.1111/bcp.12314
Prevalence, nature and potential preventability of adverse drug events - a population-based medical record study of 4970 adults
Abstract
Aims: To estimate the 3 month prevalence of adverse drug events (ADEs), categories of ADEs and preventable ADEs, and the preventability of ADEs among adults in Sweden. Further, to identify drug classes and organ systems associated with ADEs and estimate their seriousness.
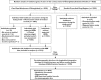
Methods: A random sample of 5025 adults in a Swedish county council in 2008 was drawn from the Total Population Register. All their medical records in 29 inpatient care departments in three hospitals, 110 specialized outpatient clinics and 51 primary care units were reviewed retrospectively in a stepwise manner, and complemented with register data on dispensed drugs. ADEs, including adverse drug reactions (ADRs), sub-therapeutic effects of drug therapy (STEs), drug dependence and abuse, drug intoxications from overdose, and morbidities due to drug-related untreated indication, were detected during a 3 month study period, and assessed for preventability.
Results: Among 4970 included individuals, the prevalence of ADEs was 12.0% (95% confidence interval (CI) 11.1, 12.9%), and preventable ADEs 5.6% (95% CI 5.0, 6.2%). ADRs (6.9%; 95% CI 6.2, 7.6%) and STEs (6.4%; 95% CI 5.8, 7.1%) were more prevalent than the other ADEs. Of the ADEs, 38.8% (95% CI 35.8-41.9%) was preventable, varying by ADE category and seriousness. ADEs were frequently associated with nervous system and cardiovascular drugs, but the associated drugs and affected organs varied by ADE category.
Conclusions: The considerable burden of ADEs and preventable ADEs from commonly used drugs across care settings warrants large-scale efforts to redesign safer, higher quality healthcare systems. The heterogeneous nature of the ADE categories should be considered in research and clinical practice for preventing, detecting and mitigating ADEs.
Keywords: adverse drug event; medical records; medication error; pharmacoepidemiology; prevalence.
© 2013 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of The British Pharmacological Society.
Figures
References
-
- Department of Health. Safety First: A Report for Patients, Clinicians and Healthcare Managers. London: Department of Health; 2006.
-
- World Health Organization. WHO Patient Safety Research – Better Knowledge for Safer Care. Geneva: World Health Organization; 2009.
-
- European Medicines Agency. Medication-Errors Workshop Report. London: European Medicines Agency; 2013.
-
- Ministry of Health and Social Affairs. National Medication Strategy. Stockholm: Ministry of Health and Social Affairs; 2011. [in Swedish]
-
- Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, Laffel G, Sweitzer BJ, Shea BF, Hallisey R. Incidence of adverse drug events and potential adverse drug events: implications for prevention. ADE Prevention Study Group. JAMA. 1995;274:29–34. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical