Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1
- PMID: 24372553
- PMCID: PMC3904269
- DOI: 10.1111/bph.12496
Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1
Abstract
Background and purpose: Endothelial dysfunction can be detected at an early stage in the development of diabetes-related microvascular disease and is associated with accelerated endothelial senescence and ageing. Hyperglycaemia-induced oxidative stress is a major contributing factor to the development of endothelial dysfunction. Clinical data indicate that the hypoglycaemic agent, metformin, has an endothelial protective action; however, its molecular and cellular mechanisms remain elusive. In the present study, we have investigated the protective effect of metformin during hyperglycaemia-induced senescence in mouse microvascular endothelial cells (MMECs).
Experimental approach: MMECs were cultured in normal glucose (11 mM) and high glucose (HG; 40 mM) in the presence and absence of metformin (50 μM) for 72 h. The expression of sirtuin-1 (SIRT1) and senescence/apoptosis-associated markers was determined by immunoblotting and immunocyto techniques. SIRT1 expression was inhibited with appropriate siRNA.
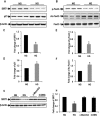
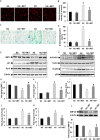
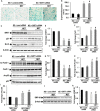
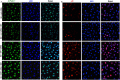
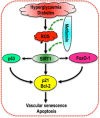
Key results: Exposure of MMECs to HG significantly reduced SIRT1 protein expression, increased forkhead box O1 (FoxO-1) and p53 acetylation, increased p21 and decreased Bcl2 expression. In addition, senescence-associated β-galactosidase activity in MMECs was increased in HG. Treatment with metformin attenuated the HG-induced reduction of SIRT1 expression, modulated the SIRT1 downstream targets FoxO-1 and p53/p21, and protected endothelial cells from HG-induced premature senescence. However, following gene knockdown of SIRT1 the effects of metformin were lost.
Conclusions and implications: HG-induced down-regulation of SIRT1 played a crucial role in diabetes-induced endothelial senescence. Furthermore, the protective effect of metformin against HG-induced endothelial dysfunction was partly due to its effects on SIRT1 expression and/or activity.
Keywords: FoxO-1; endothelial dysfunction; forkhead box O1 transcription factor; hyperglycaemia; metformin; microvascular endothelial cells; reactive oxygen species; sirtuin1; vascular senescence.
© 2013 The Authors. British Journal of Pharmacology published by John Wiley &. Sons Ltd on behalf of The British Pharmacological Society.
Figures


References
-
- Bai B, Liang Y, Xu C, Lee MY, Xu A, Wu D, et al. Cyclin-dependent kinase 5-mediated hyperphosphorylation of sirtuin-1 contributes to the development of endothelial senescence and atherosclerosis. Circulation. 2012;126:729–740. - PubMed
-
- Balestrieri ML, Rienzo M, Felice F, Rossiello R, Grimaldi V, Milone L, et al. High glucose downregulates endothelial progenitor cell number via SIRT1. Biochim Biophys Acta. 2008;1784:936–945. - PubMed
-
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

