Dynamic Epstein-Barr virus gene expression on the path to B-cell transformation
- PMID: 24373315
- PMCID: PMC4911173
- DOI: 10.1016/B978-0-12-800098-4.00006-4
Dynamic Epstein-Barr virus gene expression on the path to B-cell transformation
Abstract
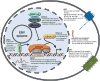
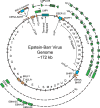
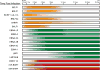
Epstein-Barr virus (EBV) is an oncogenic human herpesvirus in the γ-herpesvirinae subfamily that contains a 170-180kb double-stranded DNA genome. In vivo, EBV commonly infects B and epithelial cells and persists for the life of the host in a latent state in the memory B-cell compartment of the peripheral blood. EBV can be reactivated from its latent state, leading to increased expression of lytic genes that primarily encode for enzymes necessary to replicate the viral genome and structural components of the virion. Lytic cycle proteins also aid in immune evasion, inhibition of apoptosis, and the modulation of other host responses to infection. In vitro, EBV has the potential to infect primary human B cells and induce cellular proliferation to yield effectively immortalized lymphoblastoid cell lines, or LCLs. EBV immortalization of B cells in vitro serves as a model system for studying EBV-mediated lymphomagenesis. While much is known about the steady-state viral gene expression within EBV-immortalized LCLs and other EBV-positive cell lines, relatively little is known about the early events after primary B-cell infection. It was previously thought that upon latent infection, EBV only expressed the well-characterized latency-associated transcripts found in LCLs. However, recent work has characterized the early, but transient, expression of lytic genes necessary for efficient transformation and delayed responses in the known latency genes. This chapter summarizes these recent findings that show how dynamic and controlled expression of multiple EBV genes can control the activation of B cells, entry into the cell cycle, the inhibition of apoptosis, and innate and adaptive immune responses.
Keywords: EBNA; Epstein–Barr virus; Herpesvirus; LMP; Latency; Lytic; Viral gene expression; Viral transformation.
© 2014 Elsevier Inc. All rights reserved.
Figures

References
-
- Alfieri C, Birkenbach M, Kieff E. Early events in Epstein-Barr virus infection of human B lymphocytes. Virology. 1991;181:595–608. - PubMed
-
- Allday MJ, Crawford DH, Griffin BE. Epstein-Barr virus latent gene expression during the initiation of B cell immortalization. J Gen Virol. 1989;70(Pt 7):1755–1764. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

