Signaling lymphocytic activation molecule (SLAM)/SLAM-associated protein pathway regulates human B-cell tolerance
- PMID: 24373350
- PMCID: PMC4077428
- DOI: 10.1016/j.jaci.2013.10.051
Signaling lymphocytic activation molecule (SLAM)/SLAM-associated protein pathway regulates human B-cell tolerance
Abstract
Background: Signaling lymphocytic activation molecule (SLAM)-associated protein (SAP) can mediate the function of SLAM molecules, which have been proposed to be involved in the development of autoimmunity in mice.
Objective: We sought to determine whether the SLAM/SAP pathway regulates the establishment of human B-cell tolerance and what mechanisms of B-cell tolerance could be affected by SAP deficiency.
Methods: We tested the reactivity of antibodies isolated from single B cells from SAP-deficient patients with X-linked lymphoproliferative disease (XLP). The expressions of SAP and SLAM family members were assessed in human bone marrow-developing B cells. We also analyzed regulatory T (Treg) cell function in patients with XLP and healthy control subjects.
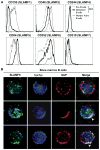
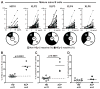
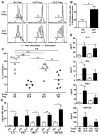
Results: We found that new emigrant/transitional B cells from patients with XLP were enriched in autoreactive clones, revealing a defective central B-cell tolerance checkpoint in the absence of functional SAP. In agreement with a B cell-intrinsic regulation of central tolerance, we identified SAP expression in a discrete subset of bone marrow immature B cells. SAP colocalized with SLAMF6 only in association with clustered B-cell receptors likely recognizing self-antigens, suggesting that SLAM/SAP regulate B-cell receptor-mediated central tolerance. In addition, patients with XLP displayed defective peripheral B-cell tolerance, which is normally controlled by Treg cells. Treg cells in patients with XLP seem functional, but SAP-deficient T cells were resistant to Treg cell-mediated suppression. Indeed, SAP-deficient T cells were hyperresponsive to T-cell receptor stimulation, which resulted in increased secretion of IL-2, IFN-γ, and TNF-α.
Conclusions: SAP expression is required for the counterselection of developing autoreactive B cells and prevents their T cell-dependent accumulation in the periphery.
Keywords: B-cell tolerance; SLAM-associated protein; regulatory T cells; signaling lymphocytic activation molecule.
Published by Mosby, Inc.
Figures




References
-
- Coffey AJ, Brooksbank RA, Brandau O, Oohashi T, Howell GR, Bye JM, et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat Genet. 1998 Oct;20(2):129–35. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] - PubMed
-
- Sayos J, Wu C, Morra M, Wang N, Zhang X, Allen D, et al. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998 Oct 1;395(6701):462–9. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] - PubMed
-
- Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol. 2011;29:665–705. [Review] - PubMed
-
- Wandstrat AE, Nguyen C, Limaye N, Chan AY, Subramanian S, Tian XH, et al. Association of extensive polymorphisms in the SLAM/CD2 gene cluster with murine lupus. Immunity. 2004 Dec;21(6):769–80. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

