Tocotrienols for normalisation of hepatic echogenic response in nonalcoholic fatty liver: a randomised placebo-controlled clinical trial
- PMID: 24373555
- PMCID: PMC3877967
- DOI: 10.1186/1475-2891-12-166
Tocotrienols for normalisation of hepatic echogenic response in nonalcoholic fatty liver: a randomised placebo-controlled clinical trial
Abstract
Background: Nonalcoholic fatty liver disease (NAFLD) is one of the commonest liver disorders. Obesity, insulin resistance, lipid peroxidation and oxidative stress have been identified amongst the possible hits leading to the onset and progression of this disease. Nutritional evaluation of NAFLD patients showed a lower-than-recommended intake of vitamin E. Vitamin E is a family of 8 isoforms, 4 tocopherols and 4 tocotrienols. Alpha-tocopherol has been widely investigated in liver diseases, whereas no previous clinical trial has investigated tocotrienols for NAFLD. Aim of the study was to determine the effects of mixed tocotrienols, in normalising the hepatic echogenic response in hypercholesterolaemic patients with ultrasound-proven NAFLD.
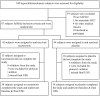
Methods: Eighty-seven untreated hypercholesterolaemic adults with ultrasound-proven NAFLD were enrolled and randomised into control group (n = 44) and tocotrienols group (n = 43). The treatment, either mixed tocotrienols 200 mg twice daily or placebo, had a 1-year duration.Normalisation of hepatic echogenic response, being the trial primary aim, was used in sample size calculations. The data were assessed according to intention to treat principle as primary outcome. Per protocol analysis was also carried out as secondary outcome measurement.
Results: Thirty and 34 participants concluded the study in the tocotrienols and placebo group respectively. Alpha-tocopherol levels were within the normal range for all subjects. As primary outcome, the normalisation of hepatic echogenic response was significantly higher for the tocotrienols treated group compared to the placebo group in the intention to treat analysis (P = 0.039; 95% CI = 0.896-6.488). As secondary objective, the per protocol assessment also showed significant rate of remission (P = 0.014; 95% CI = 1.117-9.456). Worsening of NAFLD grade was recorded in two patients in the placebo group, but none in the group treated with tocotrienols. No adverse events were reported for both groups.
Conclusion: This is the first clinical trial that showed the hepatoprotective effects of mixed palm tocotrienols in hypercholesterolemic adults with NAFLD.
Trial registration: ClinicalTrials.gov NCT00753532.
Figures
References
-
- Albano E, Mottaran E, Occhino G, Reale E, Vidali M. Review article: role of oxidative stress in the progression of non-alcoholic steatosis. Aliment Pharmacol Ther. 2005;12(Suppl. 2):71–73. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials