Measuring the dynamic longitudinal cortex development in infants by reconstruction of temporally consistent cortical surfaces
- PMID: 24374075
- PMCID: PMC3951558
- DOI: 10.1016/j.neuroimage.2013.12.038
Measuring the dynamic longitudinal cortex development in infants by reconstruction of temporally consistent cortical surfaces
Abstract
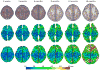
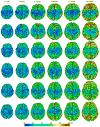
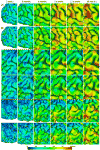
Quantitative measurement of the dynamic longitudinal cortex development during early postnatal stages is of great importance to understand the early cortical structural and functional development. Conventional methods usually reconstruct the cortical surfaces of longitudinal images from the same subject independently, which often generate longitudinally-inconsistent cortical surfaces and thus lead to inaccurate measurement of cortical changes, especially for vertex-wise mapping of cortical development. This paper aims to address this problem by presenting a method to reconstruct temporally-consistent cortical surfaces from longitudinal infant brain MR images, for accurate and consistent measurement of the dynamic cortex development in infants. Specifically, the longitudinal development of the inner cortical surface is first modeled by a deformable growth sheet with elasto-plasticity property to establish longitudinally smooth correspondences of the inner cortical surfaces. Then, the modeled longitudinal inner cortical surfaces are jointly deformed to locate both inner and outer cortical surfaces with a spatial-temporal deformable surface method. The method has been applied to 13 healthy infants, each with 6 serial MR scans acquired at 2 weeks, 3 months, 6 months, 9 months, 12 months and 18 months of age. Experimental results showed that our method with the incorporated longitudinal constraints can reconstruct the longitudinally-dynamic cortical surfaces from serial infant MR images more consistently and accurately than the previously published methods. By using our method, for the first time, we can characterize the vertex-wise longitudinal cortical thickness development trajectory at multiple time points in the first 18 months of life. Specifically, we found the highly age-related and regionally-heterogeneous developmental trajectories of the cortical thickness during this period, with the cortical thickness increased most from 3 to 6 months (16.2%) and least from 9 to 12 months (less than 0.1%). Specifically, the central sulcus only underwent significant increase of cortical thickness from 6 to 9 months and the occipital cortex underwent significant increase from 0 to 9 months, while the frontal, temporal and parietal cortices grew continuously in this first 18 months of life. The adult-like spatial patterns of cortical thickness were generally present at 18 months of age. These results provided detailed insights into the dynamic trajectory of the cortical thickness development in infants.
Keywords: Cortical folding; Cortical surface reconstruction; Cortical thickness; Infant; Longitudinal development.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
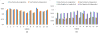

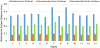

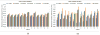
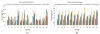

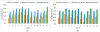

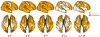
References
Publication types
MeSH terms
Grants and funding
- R01 EB006733/EB/NIBIB NIH HHS/United States
- R01 AG041721/AG/NIA NIH HHS/United States
- EB008374/EB/NIBIB NIH HHS/United States
- MH088520/MH/NIMH NIH HHS/United States
- AG042599/AG/NIA NIH HHS/United States
- R01 NS055754/NS/NINDS NIH HHS/United States
- EB008760/EB/NIBIB NIH HHS/United States
- EB009634/EB/NIBIB NIH HHS/United States
- R01 MH070890/MH/NIMH NIH HHS/United States
- U01 MH070890/MH/NIMH NIH HHS/United States
- NS055754/NS/NINDS NIH HHS/United States
- MH064065/MH/NIMH NIH HHS/United States
- MH070890/MH/NIMH NIH HHS/United States
- R01 EB008374/EB/NIBIB NIH HHS/United States
- MH100217/MH/NIMH NIH HHS/United States
- HD053000/HD/NICHD NIH HHS/United States
- R03 EB008760/EB/NIBIB NIH HHS/United States
- R01 AG042599/AG/NIA NIH HHS/United States
- P50 MH064065/MH/NIMH NIH HHS/United States
- R01 EB009634/EB/NIBIB NIH HHS/United States
- RC1 MH088520/MH/NIMH NIH HHS/United States
- R01 HD053000/HD/NICHD NIH HHS/United States
- AG041721/AG/NIA NIH HHS/United States
- EB006733/EB/NIBIB NIH HHS/United States
- R01 MH100217/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

