Antiviral strategies for hepatitis E virus
- PMID: 24374149
- PMCID: PMC7113752
- DOI: 10.1016/j.antiviral.2013.12.005
Antiviral strategies for hepatitis E virus
Abstract
The hepatitis E virus is a common cause of acute hepatitis. Contrary to hepatitis B and C, hepatitis E is mostly a mild infection, although it has a high mortality in pregnant women and can evolve to chronicity in immunocompromised patients. Ribavirin and pegylated interferon-α are the only available therapies, but both have side effects that are not acceptable for prophylaxis or treatment of mild infections. In addition, these drugs cannot be used for all patient types (e.g. in case of pregnancy, specific organ transplants or co-morbidities) and in resource-poor settings. Hence there is an urgent need for better antiviral treatments that are efficacious and safe, also during pregnancy. In this review, a concise introduction to the virus and disease is provided, followed by a discussion of the available assay systems and potential molecular targets (viral proteins and host factors) for the development of inhibitors of HEV replication. Finally, directions for future research are presented.
Keywords: Antiviral therapy; Hepatitis E virus; Host factor; Inhibitor; Interferon; Ribavirin.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures

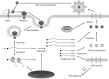

References
-
- Agrawal S., Gupta D., Panda S.K. The 3′ end of hepatitis E virus (HEV) genome binds specifically to the viral RNA-dependent RNA polymerase (RdRp) Virology. 2001;282:87–101. - PubMed
-
- Ahmed J.A., Moturi E., Spiegel P., Schilperoord M., Burton W., Kassim N.H., Mohamed A., Ochieng M., Nderitu L., Navarro-Colorado C., Burke H., Cookson S., Handzel T., Waiboci L.W., Montgomery J.M., Teshale E., Marano N. Hepatitis E outbreak, Dadaab refugee camp, Kenya, 2012. Emerg. Infect. Dis. 2013;19:1010–1012. - PMC - PubMed
-
- Alric L., Bonnet D., Laurent G., Kamar N., Izopet J. Chronic hepatitis E virus infection: successful virologic response to pegylated interferon-alpha therapy. Ann. Intern. Med. 2010;153:135–136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

