Molecular dialogs between the ischemic brain and the peripheral immune system: dualistic roles in injury and repair
- PMID: 24374228
- PMCID: PMC4014303
- DOI: 10.1016/j.pneurobio.2013.12.002
Molecular dialogs between the ischemic brain and the peripheral immune system: dualistic roles in injury and repair
Abstract
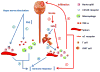
Immune and inflammatory responses actively modulate the pathophysiological processes of acute brain injuries such as stroke. Soon after the onset of stroke, signals such as brain-derived antigens, danger-associated molecular patterns (DAMPs), cytokines, and chemokines are released from the injured brain into the systemic circulation. The injured brain also communicates with peripheral organs through the parasympathetic and sympathetic branches of the autonomic nervous system. Many of these diverse signals not only activate resident immune cells in the brain, but also trigger robust immune responses in the periphery. Peripheral immune cells then migrate toward the site of injury and release additional cytokines, chemokines, and other molecules, causing further disruptive or protective effects in the ischemic brain. Bidirectional communication between the injured brain and the peripheral immune system is now known to regulate the progression of stroke pathology as well as tissue repair. In the end, this exquisitely coordinated crosstalk helps determine the fate of animals after stroke. This article reviews the literature on ischemic brain-derived signals through which peripheral immune responses are triggered, and the potential impact of these peripheral responses on brain injury and repair. Pharmacological strategies and cell-based therapies that target the dialog between the brain and peripheral immune system show promise as potential novel treatments for stroke.
Keywords: Brain repair; Cerebral ischemic injury; Inflammation; Innate immunity.
Published by Elsevier Ltd.
Conflict of interest statement
Figures


References
-
- Adami C, Bianchi R, Pula G, Donato R. S100B-stimulated NO production by BV-2 microglia is independent of RAGE transducing activity but dependent on RAGE extracellular domain. Biochim Biophys Acta. 2004;1742:169–177. - PubMed
-
- Albini A, Marchisone C, Del GF, Benelli R, Masiello L, Tacchetti C, Bono M, Ferrantini M, Rozera C, Truini M, Belardelli F, Santi L, Noonan DM. Inhibition of angiogenesis and vascular tumor growth by interferon-producing cells: A gene therapy approach. Am J Pathol. 2000;156:1381–1393. - PMC - PubMed
-
- Amantea D, Nappi G, Bernardi G, Bagetta G, Corasaniti MT. Post-ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J. 2009;276:13–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

