Altered profile of human gut microbiome is associated with cirrhosis and its complications
- PMID: 24374295
- PMCID: PMC3995845
- DOI: 10.1016/j.jhep.2013.12.019
Altered profile of human gut microbiome is associated with cirrhosis and its complications
Abstract
Background & aims: The gut microbiome is altered in cirrhosis; however its evolution with disease progression is only partly understood. We aimed to study changes in the microbiome over cirrhosis severity, its stability over time and its longitudinal alterations with decompensation.
Methods: Controls and age-matched cirrhotics (compensated/decompensated/hospitalized) were included. Their stool microbiota was quantified using multi-tagged pyrosequencing. The ratio of autochthonous to non-autochthonous taxa was calculated as the cirrhosis dysbiosis ratio (CDR); a low number indicating dysbiosis. Firstly, the microbiome was compared between controls and cirrhotic sub-groups. Secondly, for stability assessment, stool collected twice within 6months in compensated outpatients was analyzed. Thirdly, changes after decompensation were assessed using (a) longitudinal comparison in patients before/after hepatic encephalopathy development (HE), (b) longitudinal cohort of hospitalized infected cirrhotics MELD-matched to uninfected cirrhotics followed for 30days.
Results: 244 subjects [219 cirrhotics (121 compensated outpatients, 54 decompensated outpatients, 44 inpatients) and 25 age-matched controls] were included. CDR was highest in controls (2.05) followed by compensated (0.89), decompensated (0.66), and inpatients (0.32, p<0.0001) and negatively correlated with endotoxin. Microbiota and CDR remained unchanged in stable outpatient cirrhotics (0.91 vs. 0.86, p=0.45). In patients studied before/after HE development, dysbiosis occurred post-HE (CDR: 1.2 to 0.42, p=0.03). In the longitudinal matched-cohort, microbiota were significantly different between infected/uninfected cirrhotics at baseline and a low CDR was associated with death and organ failures within 30days.
Conclusions: Progressive changes in the gut microbiome accompany cirrhosis and become more severe in the setting of decompensation. The cirrhosis dysbiosis ratio may be a useful quantitative index to describe microbiome alterations accompanying cirrhosis progression.
Keywords: Acute-on-chronic liver failure; Cirrhosis dysbiosis ratio; Decompensation; Endotoxin; Hepatic encephalopathy; Infections; MELD score; Microbiota.
Copyright © 2013 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures
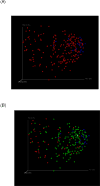

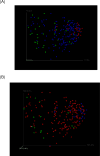
References
-
- Merli M, Lucidi C, Giannelli V, Giusto M, Riggio O, Falcone M, et al. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol. 2010;8:979–985. - PubMed
-
- Quigley EM, Stanton C, Murphy EF. The gut microbiota and the liver. Pathophysiological and clinical implications. J Hepatol. 2013;58:1020–1027. - PubMed
-
- Wiest R, Krag A, Gerbes A. Spontaneous bacterial peritonitis: recent guidelines and beyond. Gut. 2012;61:297–310. - PubMed
-
- Arvaniti V, D'Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–1256. 1256 e1241-1245. - PubMed
-
- Bajaj JS, O'Leary JG, Reddy KR, Wong F, Olson JC, Subramanian RM, et al. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology. 2012;56:2328–2335. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

