Effects of sustained proNGF blockade on attentional capacities in aged rats with compromised cholinergic system
- PMID: 24374328
- PMCID: PMC3945974
- DOI: 10.1016/j.neuroscience.2013.12.042
Effects of sustained proNGF blockade on attentional capacities in aged rats with compromised cholinergic system
Abstract
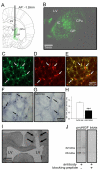
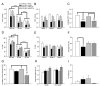
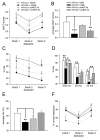
Disruption in nerve growth factor (NGF) signaling via tropomyosin-related kinase A (trkA) receptors compromises the integrity of the basal forebrain (BF) cholinergic system, yielding cognitive, specifically attentional, impairments in Alzheimer's disease (AD). Although normal aging is considered a risk factor for AD, the mechanisms underlying the selective vulnerability of the aging cholinergic system to trkA disruption is not clear. The levels of proNGF, a proneurotrophin that possesses higher affinity for p75 receptors, increase in aging. The present study was designed to test the hypothesis that cholinergic and attentional dysfunction in aged rats with reduced BF trkA receptors occurs due to the overactivation of endogenous proNGF signaling. We employed a viral vector that produced trkA shRNA to suppress trkA receptors in the corticopetal cholinergic neurons of aged rats. BF trkA suppression impaired animals' performance on signal trials in both the sustained attention task (SAT) and the cognitively taxing distractor version of SAT (dSAT) and these deficits were normalized by chronic intracerebroventricular administration of proNGF antibody. Moreover, depolarization-evoked acetylcholine (ACh) release and the density of cortical cholinergic fibers were partially restored in these animals. However, SAT/dSAT scores reflecting overall performance did not improve following proNGF blockade in trkA knockdown rats due to impaired performance in non-signal trials. Sustained proNGF blockade alone did not alter baseline attentional performance but produced moderate impairments during challenging conditions. Collectively, our findings indicate that barring proNGF-p75 signaling may exert some beneficial effects on attentional capacities specifically when BF trkA signaling is abrogated. However, endogenous proNGF may also possess neurotrophic effects and blockade of this proneurotrophin may not completely ameliorate attentional impairments in AD and potentially hinder performance during periods of high cognitive load in normal aging.
Keywords: Alzheimer’s disease; acetylcholine; aging; attention; proNGF.
Copyright © 2013 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures

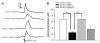

Similar articles
-
Developmental suppression of forebrain trkA receptors and attentional capacities in aging rats: A longitudinal study.Behav Brain Res. 2017 Sep 29;335:111-121. doi: 10.1016/j.bbr.2017.08.017. Epub 2017 Aug 10. Behav Brain Res. 2017. PMID: 28803853 Free PMC article.
-
Diminished trkA receptor signaling reveals cholinergic-attentional vulnerability of aging.Eur J Neurosci. 2013 Jan;37(2):278-93. doi: 10.1111/ejn.12090. Epub 2012 Dec 11. Eur J Neurosci. 2013. PMID: 23228124 Free PMC article.
-
Interactions between Aβ oligomers and presynaptic cholinergic signaling: age-dependent effects on attentional capacities.Behav Brain Res. 2014 Nov 1;274:30-42. doi: 10.1016/j.bbr.2014.07.046. Epub 2014 Aug 4. Behav Brain Res. 2014. PMID: 25101540 Free PMC article.
-
Effects of Reactive Oxygen and Nitrogen Species on TrkA Expression and Signalling: Implications for proNGF in Aging and Alzheimer's Disease.Cells. 2021 Aug 4;10(8):1983. doi: 10.3390/cells10081983. Cells. 2021. PMID: 34440751 Free PMC article. Review.
-
NGF-cholinergic dependency in brain aging, MCI and Alzheimer's disease.Curr Alzheimer Res. 2007 Sep;4(4):351-8. doi: 10.2174/156720507781788774. Curr Alzheimer Res. 2007. PMID: 17908036 Review.
Cited by
-
Effect of red and black ginseng on cholinergic markers, presynaptic markers, and neurotrophins in the brain of aged mice.Food Sci Biotechnol. 2017 Oct 16;26(6):1743-1747. doi: 10.1007/s10068-017-0235-7. eCollection 2017. Food Sci Biotechnol. 2017. PMID: 30263713 Free PMC article.
-
Transcriptomic changes in the prefrontal cortex of rats as a function of age and cognitive engagement.Neurobiol Learn Mem. 2019 Sep;163:107035. doi: 10.1016/j.nlm.2019.107035. Epub 2019 Jun 8. Neurobiol Learn Mem. 2019. PMID: 31185277 Free PMC article.
-
Reduction of acetylcholine in the hippocampus of hippocampal cholinergic neurostimulating peptide precursor protein knockout mice.Sci Rep. 2021 Nov 11;11(1):22072. doi: 10.1038/s41598-021-01667-8. Sci Rep. 2021. PMID: 34764402 Free PMC article.
-
Developmental suppression of forebrain trkA receptors and attentional capacities in aging rats: A longitudinal study.Behav Brain Res. 2017 Sep 29;335:111-121. doi: 10.1016/j.bbr.2017.08.017. Epub 2017 Aug 10. Behav Brain Res. 2017. PMID: 28803853 Free PMC article.
-
The Intersection of NGF/TrkA Signaling and Amyloid Precursor Protein Processing in Alzheimer's Disease Neuropathology.Int J Mol Sci. 2017 Jun 20;18(6):1319. doi: 10.3390/ijms18061319. Int J Mol Sci. 2017. PMID: 28632177 Free PMC article. Review.
References
-
- Al-Shawi R, Hafner A, Chun S, Raza S, Crutcher K, Thrasivoulou C, Simons P, Cowen T. ProNGF, sortilin, and age-related neurodegeneration. Ann N Y Acad Sci. 2007;1119:208–215. - PubMed
-
- Al-Shawi R, Hafner A, Olsen J, Olson J, Chun S, Raza S, Thrasivoulou C, Lovestone S, Killick R, Simons P, Cowen T. Neurotoxic and neurotrophic roles of proNGF and the receptor sortilin in the adult and ageing nervous system. Eur J Neurosci. 2008;27:2103–2114. - PubMed
-
- Arnold HM, Burk JA, Hodgson EM, Sarter M, Bruno JP. Differential cortical acetylcholine release in rats performing a sustained attention task versus behavioral control tasks that do not explicitly tax attention. Neuroscience. 2002;114:451–460. - PubMed
-
- Bruno MA, Leon WC, Fragoso G, Mushynski WE, Almazan G, Cuello AC. Amyloid beta- induced nerve growth factor dysmetabolism in Alzheimer disease. J Neuropathol Exp Neurol. 2009;68:857–869. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

