Long-term follow-up of study participants from prophylactic HIV vaccine clinical trials in Africa
- PMID: 24374365
- PMCID: PMC4130282
- DOI: 10.4161/hv.27559
Long-term follow-up of study participants from prophylactic HIV vaccine clinical trials in Africa
Abstract
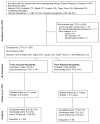
Long-term safety is critical for the development and later use of a vaccine to prevent HIV/AIDS. Likewise, the persistence of vaccine-induced antibodies and their impact on HIV testing must be established. IAVI has sponsored several Phase I and IIA HIV vaccine trials enrolling healthy, HIV-seronegative African volunteers. Plasmid DNA and viral vector based vaccines were tested. No vaccine-related serious adverse events were reported. After completion of vaccine trials conducted between 2001-2007, both vaccine and placebo recipients were offered enrolment into an observational long-term follow-up study (LTFU) to monitor potential late health effects and persistence of immune responses. At scheduled 6-monthly clinic visits, a health questionnaire was administered; clinical events were recorded and graded for severity. Blood was drawn for HIV testing and cellular immune assays. 287 volunteers were enrolled; total follow-up after last vaccination was 1463 person years (median: 5.2 years). Ninety-three (93)% of volunteers reported good health at their last LTFU visit. Infectious diseases and injuries accounted for almost 50% of the 175 reported clinical events, of which over 95% were mild or moderate in severity. There were 30 six pregnancies, six incident HIV infections and 14 volunteers reported cases of social harm. Persistence of immune responses was rare. No safety signal was identified. No potentially vaccine-related medical condition, no immune mediated disease, or malignancy was reported. HIV vaccines studied in these trials had a low potential of induction of persisting HIV antibodies.
Keywords: Africa; HIV vaccine trials; healthy adult volunteers; long term follow-up; safety.
Figures

References
-
- Peters BS, Jaoko W, Vardas E, Panayotakopoulos G, Fast P, Schmidt C, Gilmour J, Bogoshi M, Omosa-Manyonyi G, Dally L, et al. Studies of a prophylactic HIV-1 vaccine candidate based on modified vaccinia virus Ankara (MVA) with and without DNA priming: effects of dosage and route on safety and immunogenicity. Vaccine. 2007;25:2120–7. doi: 10.1016/j.vaccine.2006.11.016. - DOI - PubMed
-
- Jaoko W, Nakwagala FN, Anzala O, Manyonyi GO, Birungi J, Nanvubya A, Bashir F, Bhatt K, Ogutu H, Wakasiaka S, et al. Safety and immunogenicity of recombinant low-dosage HIV-1 A vaccine candidates vectored by plasmid pTHr DNA or modified vaccinia virus Ankara (MVA) in humans in East Africa. Vaccine. 2008;26:2788–95. doi: 10.1016/j.vaccine.2008.02.071. - DOI - PubMed
-
- Vardas E, Kaleebu P, Bekker LG, Hoosen A, Chomba E, Johnson PR, Anklesaria P, Birungi J, Barin B, Boaz M, et al. A phase 2 study to evaluate the safety and immunogenicity of a recombinant HIV type 1 vaccine based on adeno-associated virus. AIDS Res Hum Retroviruses. 2010;26:933–42. doi: 10.1089/aid.2009.0242. - DOI - PubMed
-
- Jaoko W, Karita E, Kayitenkore K, Omosa-Manyonyi G, Allen S, Than S, Adams EM, Graham BS, Koup RA, Bailer RT, et al. Safety and immunogenicity study of Multiclade HIV-1 adenoviral vector vaccine alone or as boost following a multiclade HIV-1 DNA vaccine in Africa. PLoS One. 2010;5:e12873. doi: 10.1371/journal.pone.0012873. - DOI - PMC - PubMed
-
- Gilbert PB, Chiu Y-L, Allen M, Lawrence DN, Chapdu C, Israel H, Holman D, Keefer MC, Wolff M, Frey SE, NIAID HIV Vaccine Trials Network Long-term safety analysis of preventive HIV-1 vaccines evaluated in AIDS vaccine evaluation group NIAID-sponsored Phase I and II clinical trials. Vaccine. 2003;21:2933–47. doi: 10.1016/S0264-410X(03)00158-0. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
