Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis
- PMID: 24374462
- PMCID: PMC12050155
- DOI: 10.1002/14651858.CD008623.pub2
Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis
Abstract
Background: Non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) are common causes of elevated liver enzymes in the general population. NASH and to some extent NAFLD have been associated with increased liver-related and all-cause mortality. No effective treatment is yet available. Recent reports have shown that the use of hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins) in patients with elevated plasma aminotransferases may result in normalisation of these liver enzymes. Whether this is a consistent effect or whether it can lead to improved clinical outcomes beyond normalisation of abnormal liver enzymes is not clear.
Objectives: To assess the beneficial and harmful effects of statins (that is, lovastatin, atorvastatin, simvastatin, pravastatin, rosuvastatin, and fluvastatin) on all-cause and liver-related mortality, adverse events, and histological, biochemical, and imaging responses in patients with NAFLD or NASH.
Search methods: We performed a computerised literature search in the Cochrane Hepato-Biliary Group Controlled Trials Register, Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded up to March 2013. We did fully recursive searches from the reference lists of all retrieved relevant publications to ensure a complete and comprehensive search of the published literature. We did not apply any restrictions regarding language of publication or publication date.
Selection criteria: All randomised clinical trials using statins as the primary treatment for NAFLD or NASH versus no treatment, placebo, or other hypolipidaemic agents.
Data collection and analysis: Data were extracted, and risk of bias of each trial was assessed independently by two or more review authors. Meta-analyses were performed whenever possible. Review Manager 5.2 was used.
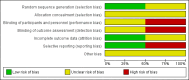
Main results: When the described search method was used and the eligibility criteria of the search results were applied, 653 records were found. Only two of these were randomised clinical trials that were considered eligible for inclusion. We assessed both trials as trials with high risk of bias. One of the trials was a pilot trial in which 16 participants with biopsy-proven NASH were randomised to receive simvastatin 40 mg (n = 10) or placebo (n = 6) once daily for 12 months. No statistically significant improvement in the aminotransferase level was seen in the simvastatin group compared with the placebo group. Liver histology was not significantly affected by simvastatin.The other trial had three arms. The trial compared atorvastatin 20 mg daily (n = 63) versus fenofibrate 200 mg daily (n = 62) versus a group treated with a combination of the two interventions (n = 61). There were no statistically significant differences between any of the three intervention groups regarding the week 54 mean activity levels of aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transpeptidase, and alkaline phosphatase. The triglyceride levels seemed higher in the fenofibrate group compared with the atorvastatin group. Liver histology was not assessed in this trial. The presence of biochemical and ultrasonographic evidence of NAFLD seemed to be higher in the fenofibrate group compared with the atorvastatin group (58% versus 33%). Three patients discontinued treatment due to myalgia and elevated serum creatine kinase activity; one from the atorvastatin group and two from the combination group. Another patient from the atorvastatin group discontinued treatment due to alanine aminotransferase activity that was over three times the upper normal limit.No data for all-cause mortality and hepatic-related mortality were reported in the included trials.
Authors' conclusions: Based on the findings of this review, which included two trials with high risk of bias and a small numbers of participants, it seems possible that statins may improve serum aminotransferase levels as well as ultrasound findings. Neither of the trials reported on possible histological changes, liver-related morbidity or mortality. Trials with larger sample sizes and low risk of bias are necessary before we may suggest statins as an effective treatment for patients with NASH. However, as statins can improve the adverse outcomes of other conditions commonly associated with NASH (for example, hyperlipidaemia, diabetes mellitus, metabolic syndrome), their use in patients with non-alcoholic steatohepatitis may be justified.
Conflict of interest statement
None known.
Figures
Update of
References
References to studies included in this review
Athyros 2006 {published data only}
-
- Athyros VG, Mikhailidis DP, Didangelos TP, Giouleme OI, Liberopoulos EN, Karagiannis A, et al. Effect of multifactorial treatment on non‐alcoholic fatty liver disease in metabolic syndrome: a randomised study. Current Medical Research and Opinion 2006;22(5):873‐83. - PubMed
Nelson 2009 {published data only}
-
- Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: a randomised placebo controlled trial. Journal of Clinical Gastroenterology 2009;43(10):990‐4. - PubMed
References to studies excluded from this review
Antonopoulos 2006 {published data only}
-
- Antonopoulos S, Mikros S, Mylonopoulou M, Kokkoris S, Giannoulis G. Rosuvastatin as a novel treatment of non‐alcoholic fatty liver disease in hyperlipidaemic patients. Atherosclerosis 2006;184:233‐4. - PubMed
Athyros 2010 {published data only}
-
- Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, et al. Safety and efficacy of long‐term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) study: a post‐hoc analysis. Lancet 2010;376:1916‐22. - PubMed
Carnelutti 2012 {published data only}
-
- Carnelutti A, Donnini D, Nadalutti G, Luca L, Cappello D, Cugini F, et al. Effect of statin therapy vs diet in hypercholesterolaemia patients affected by nonalcoholic steatohepatitis (NASH). Digestive and Liver Disease 2012;44S:S25.
Gomez 2006 {published data only}
-
- Gomez‐Dominguez E, Gisbert JP, Moreno‐ Monteagudo JA, Garcia‐Buey L, Moreno‐Otero R. A pilot study of atorvastatin treatment in dyslipemic, non‐alcoholic fatty liver patients. Alimentary Pharmacology and Therapeutics 2006;23:1643‐7. - PubMed
Han 2012 {published data only}
-
- Han KH, Rha SW, Kang HJ, Bae JW, Choi BJ, Choi SY, et al. Evaluation of short‐term safety and efficacy of HMG‐CoA reductase inhibitors in hypercholesterolemic patients with elevated serum alanine transaminase concentrations: PITCH study (PITavastatin versus atorvastatin to evaluate the effect on patients with hypercholesterolemia and mild to moderate hepatic damage). Journal of Clinical Lipidology 2012;6(4):340‐51. - PubMed
Harlander 2001 {published data only}
-
- Harlander JC, Kwo PY, Cummings OW. Atorvastatin for the treatment of NASH. Gastroenterology 2001;120A:544.
Hatzitolios 2004 {published data only}
-
- Hatzitolios A, Savopoulos C, Lazaraki G, Sidiropoulos I, Haritanti P, Lefkopoulos A, et al. Efficacy of omega‐3 fatty acids, atorvastatin and orlistat in non‐alcoholic fatty liver disease with dyslipidaemia. Indian Journal of Gastroenterology 2004;23:131‐4. - PubMed
Kiyici 2003 {published data only}
-
- Kiyici M, Gulten M, Gurel S, Nak SG, Dolar E, Savci G, et al. Ursodeoxycholic acid and atorvastatin in the treatment of nonalcoholic steatohepatitis. Canadian Journal of Gastroenterology 2003;17(12):713‐8. - PubMed
Lewis 2007 {published data only}
-
- Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R, et al. Efficacy and safety of high‐dose pravastatin in hypercholesteraemic patients with well compensated chronic liver disease: results of a prospective, randomised, double blind, placebo‐controlled, multicenter trial. Hepatology 2007;46(5):1453‐63. - PubMed
Rallidis 2004 {published data only}
-
- Rallidis LS, Drakoulis CK, Parasi AS. Pravastatin in patients with nonalcoholic steatohepatitis: results of a pilot study. Atherosclerosis 2004;174:193‐6. - PubMed
Tavakkoli 2009 {published data only}
-
- Tavakkoli H, Adilipour H, Ghaemaghami Z, Minakari M, Adibi P. Simvastatin in treatment of non‐alcoholic steatohepatitis: a clinical trial. Govaresh 2009;14:28.
Additional references
AGA 2012
-
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non‐alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012;142:1592–609. - PubMed
Ahmed 2006
-
- Ahmed MH. Rosuvastatin: a safe and effective treatment for dyslipidaemia associated with non‐alcoholic steatohepatitis (NASH). Scandinavian Journal of Gastroenterology 2006;41(5):631. - PubMed
Athyros 2005
-
- Athyros VG, Mikhailidis DP, Papageorgiou AA, Didangelos TP, Peletidou A, Kleta D, et al. Targeting vascular risk in patients with metabolic syndrome but without diabetes. Metabolism 2005;54:1065‐74. - PubMed
Bacon 1994
-
- Bacon BR, Faravash MJ, Janney CG, Neuschwander‐Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology 1994;107:1103‐9. - PubMed
Ballantyne 2003
-
- Ballantyne CM, Corsini A, Davidson MH, Holdaas H, Jacobson TA, Leitersdorf E, et al. Risk for myopathy with statin therapy in high‐risk patients. Archives of Internal Medicine 2003;163:553‐64. - PubMed
Brunt 1999
-
- Brunt EM, Janney CG, Bisceglie AM, Neuschwander‐Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. American Journal of Gastroenterology 1999;94:2467‐74. - PubMed
Brunt 2001
-
- Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Seminars in Liver Disease 2001;21(1):3‐16. - PubMed
Brunt 2005
-
- Brunt EM. NASH: pathologic features and differential diagnosis. Seminars in Diagnostic Pathology 2005;22:330‐8. - PubMed
Brunt 2007
-
- Brunt EM. Pathology of fatty liver disease. Modern Pathology 2007;20 Suppl 1:S40. - PubMed
Cello 1990
-
- Cello JP, Grendell JH. The liver in systemic conditions. In: Zakim D, Boyer TD editor(s). Hepatology. Philadelphia: WB Saunders, 1990:1428.
Cheung 2008
-
- Cheung O, Sanyal AJ. Abnormalities of lipid metabolism in nonalcoholic fatty liver disease. Seminars in Liver Disease 2008;28(4):351‐9. - PubMed
Fujita 2009
-
- Fujita K, Nozaki Y, Wada K, Yoneda M, Fujimoto Y, Fujitake M, et al. Dysfunctional very‐low‐density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology 2009;50:772‐80. - PubMed
Gluud 2001
-
- Gluud C. Alcoholic hepatitis: no glucocorticosteroids?. In: Leuschner U, James OFW, Dancygier H editor(s). Steatohepatitis (NASH and ASH) ‐ Falk Symposium 121. Lancaster: Kluwer Academic Publisher, 2001:322‐42.
Gluud 2007
-
- Gluud C, Brok J, Gong Y, Koretz RL. Hepatology may have problems with putative surrogate outcome measures. Journal of Hepatology 2007;46(4):734‐42. - PubMed
Gluud 2013
-
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2013, Issue 11. Art. No.: LIVER.
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hsiao 2007
-
- Hsiao PJ, Kuo KK, Shin SJ, Yang YH, Lin WY, Yang JF, et al. Significant correlations between severe fatty liver and risk factors for metabolic syndrome. Journal of Gastroenterology and Hepatology 2007;22(12):2118‐23. - PubMed
ICH‐GCP 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997.
Istvan 2001
-
- Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG‐CoA reductase. Science 2001;292:1160‐4. - PubMed
Jakobsen 2013
-
- Jakobsen JC, Gluud C. The necessity of randomized clinical trials. British Journal of Medicine and Medical Research 2013;3(4):1453‐68.
Kashani 2006
-
- Kashani A, Phillips CO, Foody JM, Wang Y, Mangalmurti S, Ko DT, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation 2006;114:2788‐97. - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Kliener 2005
-
- Kleiner DE, Brunt EM, Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313‐21. - PubMed
Lee 1988
-
- Lee RG. Nonalcoholic steatohepatitis: a study of 49 patients. Human Pathology 1989;20:594‐8. - PubMed
Lomas 1991
-
- Lomas J. Words without action? The production, dissemination, and impact of consensus recommendations. Annual Review of Public Health 1991;12:41‐65. - PubMed
Ludwig 1980
-
- Ludwig J, Viggiano TR, McGill DB, Ott BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clinic Proceedings 1980;55:434‐8. - PubMed
Lundh 2012
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. - PubMed
NCEP ATP III report
-
- Executive summary of the third report of the National Cholesterol Education Program (NCEP), Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; Vol. 285:2486‐97. - PubMed
Ness 1996
-
- Ness GC, Zhao Z, Lopez D. Inhibitors of cholesterol biosynthesis increase hepatic low density lipoprotein receptor protein degradation. Archives of Biochemistry and Biophysics 1996;325:242‐8. - PubMed
Poonam 2007
-
- Poonam M, Zobair M. Abdominal ultrasound for diagnosis of nonalcoholic fatty liver disease (NAFLD). American Journal of Gastroenterology 2007;102:2716‐7. - PubMed
Powell 1990
-
- Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell W. The natural history of nonalcoholic steatohepatitis: a follow up study of forty‐two patients for up to 21 years. Hepatology 1990;11(1):74‐80. - PubMed
Puri 2007
-
- Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, et al. A lipidemic analysis of nonalcoholic fatty liver disease. Hepatology 2007;46(4):1081‐90. - PubMed
Rofsky 1995
-
- Rofsky NM, Fleshaker H. CT and MRI of diffuse liver disease. Seminars in Ultrasound, CT, and MR 1995;16:16‐33. - PubMed
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Saadeh 2002
-
- Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002;123:745‐50. - PubMed
Sanyal 2001
-
- Sanyal AJ, Campbell‐Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 2001;120(5):1183‐92. - PubMed
Savovic 2012
-
- Savovic J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. - PubMed
Savovic 2012a
-
- Savovic J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Schwenzer 2009
-
- Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non‐invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. Journal of Hepatology 2009;51(3):433‐5. - PubMed
Taylor 2013
Tobert 1988
-
- Tobert JA. Efficacy and long‐term adverse effect pattern of lovastatin. American Journal of Cardiology 1988;62:28J. - PubMed
Vale 2011
Villanueva 2009
Wang 2006
-
- Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology 2006;147(2):943‐51. - PubMed
Wei 2006
-
- Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. American Journal of Physiology, Endocrinology and Metabolism 2006;291(2):E275‐81. - PubMed
Wood 2008
Yeh 2007
-
- Yeh MM, Brunt EM. Pathology of nonalcoholic fatty liver disease. American Journal of Clinical Pathology 2007;128:837‐4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical