Maternal dexamethasone exposure during pregnancy in rats disrupts gonadotropin-releasing hormone neuronal development in the offspring
- PMID: 24374911
- PMCID: PMC3921457
- DOI: 10.1007/s00441-013-1765-9
Maternal dexamethasone exposure during pregnancy in rats disrupts gonadotropin-releasing hormone neuronal development in the offspring
Abstract
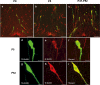
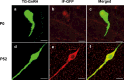
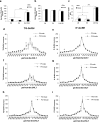
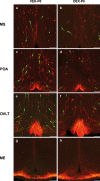
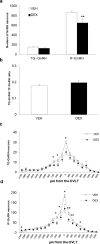
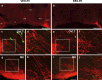
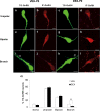
The migration of gonadotropin-releasing hormone (GnRH) neurons from the olfactory placode to the preoptic area (POA) from embryonic day 13 is important for successful reproduction during adulthood. Whether maternal glucocorticoid exposure alters GnRH neuronal morphology and number in the offspring is unknown. This study determines the effect of maternal dexamethasone (DEX) exposure on enhanced green fluorescent protein (EGFP) driven by GnRH promoter neurons (TG-GnRH) in transgenic rats dual-labelled with GnRH immunofluorescence (IF-GnRH). The TG-GnRH neurons were examined in intact male and female rats at different postnatal ages, as a marker for GnRH promoter activity. Pregnant females were subcutaneously injected with DEX (0.1 mg/kg) or vehicle daily during gestation days 13-20 to examine the number of GnRH neurons in P0 male offspring. The total number of TG-GnRH neurons and TG-GnRH/IF-GnRH neuronal ratio increased from P0 and P5 stages to P47-52 stages, suggesting temporal regulation of GnRH promoter activity during postnatal development in intact rats. In DEX-treated P0 males, the number of IF-GnRH neurons decreased within the medial septum, organum vasculosom of the lamina terminalis (OVLT) and anterior hypothalamus. The percentage of TG-GnRH neurons with branched dendritic structures decreased in the OVLT of DEX-P0 males. These results suggest that maternal DEX exposure affects the number and dendritic development of early postnatal GnRH neurons in the OVLT/POA, which may lead to altered reproductive functions in adults.
Figures
Similar articles
-
Maternal Dexamethasone Exposure Alters Synaptic Inputs to Gonadotropin-Releasing Hormone Neurons in the Early Postnatal Rat.Front Endocrinol (Lausanne). 2016 Aug 31;7:117. doi: 10.3389/fendo.2016.00117. eCollection 2016. Front Endocrinol (Lausanne). 2016. PMID: 27630615 Free PMC article.
-
Maternal dexamethasone exposure inhibits the gonadotropin-releasing hormone neuronal movement in the preoptic area of rat offspring.Dev Neurosci. 2014;36(2):95-107. doi: 10.1159/000360416. Epub 2014 Apr 3. Dev Neurosci. 2014. PMID: 24713635
-
The Effects of GABA on embryonic gonadotropin-releasing hormone neurons in rat hypothalamic primary culture.J Reprod Dev. 2007 Apr;53(2):323-31. doi: 10.1262/jrd.18103. Epub 2006 Dec 20. J Reprod Dev. 2007. PMID: 17179652
-
Gonadotropin-releasing hormone neurons, NMDA receptors, and their regulation by steroid hormones across the reproductive life cycle.Brain Res Brain Res Rev. 2001 Nov;37(1-3):235-48. doi: 10.1016/s0165-0173(01)00121-7. Brain Res Brain Res Rev. 2001. PMID: 11744089 Review.
-
Development of the gonadotropin-releasing hormone system.J Neuroendocrinol. 2022 May;34(5):e13087. doi: 10.1111/jne.13087. Epub 2022 Jan 23. J Neuroendocrinol. 2022. PMID: 35067985 Free PMC article. Review.
Cited by
-
Maternal Dexamethasone Exposure Alters Synaptic Inputs to Gonadotropin-Releasing Hormone Neurons in the Early Postnatal Rat.Front Endocrinol (Lausanne). 2016 Aug 31;7:117. doi: 10.3389/fendo.2016.00117. eCollection 2016. Front Endocrinol (Lausanne). 2016. PMID: 27630615 Free PMC article.
-
Effects of prenatal stress on reproductive function of male offspring through the KISS1 system.Endocr Connect. 2024 Sep 13;13(10):e240027. doi: 10.1530/EC-24-0027. Print 2024 Oct 1. Endocr Connect. 2024. PMID: 39140811 Free PMC article.
-
Sex- and brain region-specific patterns of gene expression associated with socially-mediated puberty in a eusocial mammal.PLoS One. 2018 Feb 23;13(2):e0193417. doi: 10.1371/journal.pone.0193417. eCollection 2018. PLoS One. 2018. PMID: 29474488 Free PMC article.
-
Early-Life Social Isolation Impairs the Gonadotropin-Inhibitory Hormone Neuronal Activity and Serotonergic System in Male Rats.Front Endocrinol (Lausanne). 2015 Nov 10;6:172. doi: 10.3389/fendo.2015.00172. eCollection 2015. Front Endocrinol (Lausanne). 2015. PMID: 26617573 Free PMC article.
References
-
- Attardi B, Tsujii T, Friedman R, Zeng Z, Roberts JL, Dellovade T, Pfaff DW, Chandran UR, Sullivan MW, DeFranco DB. Glucocorticoid repression of gonadotropin-releasing hormone gene expression and secretion in morphologically distinct subpopulations of GT1-7 cells. Mol Cell Endocrinol. 1997;131:241–255. doi: 10.1016/S0303-7207(97)00102-0. - DOI - PubMed
-
- Chandran UR, Attardi B, Friedman R, Dong KW, Roberts JL, DeFranco DB. Glucocorticoid receptor-mediated repression of gonadotropin-releasing hormone promoter activity in GT1 hypothalamic cell lines. Endocrinology. 1994;134:1467–1474. - PubMed
-
- Chandran UR, Attardi B, Friedman R, Zheng Z, Roberts JL, DeFranco DB. Glucocorticoid repression of the mouse gonadotropin-releasing hormone gene is mediated by promoter elements that are recognized by heteromeric complexes containing glucocorticoid receptor. J Biol Chem. 1996;271:20412–20420. doi: 10.1074/jbc.271.34.20412. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

