High-field small animal magnetic resonance oncology studies
- PMID: 24374985
- PMCID: PMC4389287
- DOI: 10.1088/0031-9155/59/2/R65
High-field small animal magnetic resonance oncology studies
Abstract
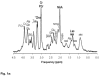
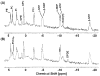
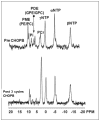
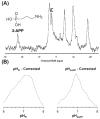
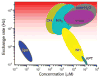
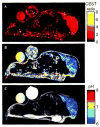
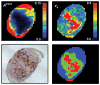
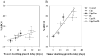
This review focuses on the applications of high magnetic field magnetic resonance imaging (MRI) and spectroscopy (MRS) to cancer studies in small animals. High-field MRI can provide information about tumor physiology, the microenvironment, metabolism, vascularity and cellularity. Such studies are invaluable for understanding tumor growth and proliferation, response to treatment and drug development. The MR techniques reviewed here include (1)H, (31)P, chemical exchange saturation transfer imaging and hyperpolarized (13)C MRS as well as diffusion-weighted, blood oxygen level dependent contrast imaging and dynamic contrast-enhanced MRI. These methods have been proven effective in animal studies and are highly relevant to human clinical studies.
Figures

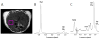






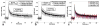
References
-
- Aboagye EO, Bhujwalla ZM. Malignant transformation alters membrane choline phospholipid metabolism of human mammary epithelial cells. Cancer Res. 1999;59:80–4. - PubMed
-
- Ackerman JJ, Grove TH, Wong GG, Gadian DG, Radda GK. Mapping of metabolites in whole animals by 31P NMR using surface coils. Nature. 1980;283:167–70. - PubMed
-
- Ackerstaff E, Pflug BR, Nelson JB, Bhujwalla ZM. Detection of increased choline compounds with proton nuclear magnetic resonance spectroscopy subsequent to malignant transformation of human prostatic epithelial cells. Cancer Res. 2001;61:3599–603. - PubMed
-
- Agris PF, Campbell ID. Proton nuclear magnetic resonance of intact Friend leukemia cells: phosphorylcholine increase during differentiation. Science. 1982;216:1325–7. - PubMed
-
- Aime S, Delli Castelli D, Terreno E. Highly sensitive MRI chemical exchange saturation transfer agents using liposomes. Angewandte Chemie. 2005;44:5513–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
