The sensor kinase DctS forms a tripartite sensor unit with DctB and DctA for sensing C4-dicarboxylates in Bacillus subtilis
- PMID: 24375102
- PMCID: PMC3957698
- DOI: 10.1128/JB.01154-13
The sensor kinase DctS forms a tripartite sensor unit with DctB and DctA for sensing C4-dicarboxylates in Bacillus subtilis
Abstract
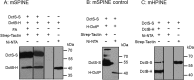
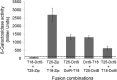
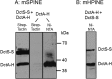
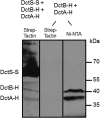
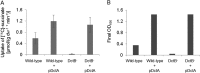
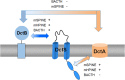
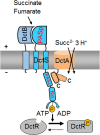
The DctSR two-component system of Bacillus subtilis controls the expression of the aerobic C4-dicarboxylate transporter DctA. Deletion of DctA leads to an increased dctA expression. The inactivation of DctB, an extracellular binding protein, is known to inhibit the expression of dctA. Here, interaction between the sensor kinase DctS and the transporter DctA as well as the binding protein DctB was demonstrated in vivo using streptavidin (Strep) or His protein interaction experiments (mSPINE or mHPINE), and the data suggest that DctA and DctB act as cosensors for DctS. The interaction between DctS and DctB was also confirmed by the bacterial two-hybrid system (BACTH). In contrast, no indication was obtained for a direct interaction between the transporter DctA and the binding protein DctB. Activity levels of uptake of [(14)C]succinate by bacteria that expressed DctA from a plasmid were similar in the absence and the presence of DctB, demonstrating that the binding protein DctB is not required for transport. Thus, DctB is involved not in transport but in cosensing with DctS, highlighting DctB as the first example of a TRAP-type binding protein that acts as a cosensor. The simultaneous presence of DctS/DctB and DctS/DctA sensor pairs and the lack of direct interaction between the cosensors DctA and DctB indicate the formation of a tripartite complex via DctS. It is suggested that the DctS/DctA/DctB complex forms the functional unit for C4-dicarboxylate sensing in B. subtilis.
Figures

Similar articles
-
Identification and characterization of a two-component sensor-kinase and response-regulator system (DcuS-DcuR) controlling gene expression in response to C4-dicarboxylates in Escherichia coli.J Bacteriol. 1999 Feb;181(4):1238-48. doi: 10.1128/JB.181.4.1238-1248.1999. J Bacteriol. 1999. PMID: 9973351 Free PMC article.
-
Roles of DctA and DctB in signal detection by the dicarboxylic acid transport system of Rhizobium leguminosarum.J Bacteriol. 1998 May;180(10):2660-9. doi: 10.1128/JB.180.10.2660-2669.1998. J Bacteriol. 1998. PMID: 9573150 Free PMC article.
-
Analysis of the C4-dicarboxylate transport genes of Rhizobium meliloti: nucleotide sequence and deduced products of dctA, dctB, and dctD.Mol Plant Microbe Interact. 1990 May-Jun;3(3):174-81. doi: 10.1094/mpmi-3-174. Mol Plant Microbe Interact. 1990. PMID: 2134335
-
C4-dicarboxylate carriers and sensors in bacteria.Biochim Biophys Acta. 2002 Jan 17;1553(1-2):39-56. doi: 10.1016/s0005-2728(01)00233-x. Biochim Biophys Acta. 2002. PMID: 11803016 Review.
-
Cooperation of Secondary Transporters and Sensor Kinases in Transmembrane Signalling: The DctA/DcuS and DcuB/DcuS Sensor Complexes of Escherichia coli.Adv Microb Physiol. 2016;68:139-67. doi: 10.1016/bs.ampbs.2016.02.003. Epub 2016 Mar 16. Adv Microb Physiol. 2016. PMID: 27134023 Review.
Cited by
-
Dynamic interaction between the CpxA sensor kinase and the periplasmic accessory protein CpxP mediates signal recognition in E. coli.PLoS One. 2014 Sep 10;9(9):e107383. doi: 10.1371/journal.pone.0107383. eCollection 2014. PLoS One. 2014. PMID: 25207645 Free PMC article.
-
A signaling complex of adenylate cyclase CyaC of Sinorhizobium meliloti with cAMP and the transcriptional regulators Clr and CycR.BMC Microbiol. 2023 Aug 26;23(1):236. doi: 10.1186/s12866-023-02989-5. BMC Microbiol. 2023. PMID: 37633907 Free PMC article.
-
Genome-based comparative analyses of Antarctic and temperate species of Paenibacillus.PLoS One. 2014 Oct 6;9(10):e108009. doi: 10.1371/journal.pone.0108009. eCollection 2014. PLoS One. 2014. PMID: 25285990 Free PMC article.
-
Protein Activity Sensing in Bacteria in Regulating Metabolism and Motility.Front Microbiol. 2020 Jan 17;10:3055. doi: 10.3389/fmicb.2019.03055. eCollection 2019. Front Microbiol. 2020. PMID: 32010106 Free PMC article. Review.
-
A sensory complex consisting of an ATP-binding cassette transporter and a two-component regulatory system controls bacitracin resistance in Bacillus subtilis.J Biol Chem. 2014 Oct 3;289(40):27899-910. doi: 10.1074/jbc.M114.596221. Epub 2014 Aug 12. J Biol Chem. 2014. PMID: 25118291 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

