NrdH Redoxin enhances resistance to multiple oxidative stresses by acting as a peroxidase cofactor in Corynebacterium glutamicum
- PMID: 24375145
- PMCID: PMC3957609
- DOI: 10.1128/AEM.03654-13
NrdH Redoxin enhances resistance to multiple oxidative stresses by acting as a peroxidase cofactor in Corynebacterium glutamicum
Abstract
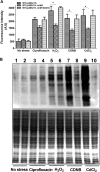
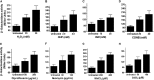
NrdH redoxins are small protein disulfide oxidoreductases behaving like thioredoxins but sharing a high amino acid sequence similarity to glutaredoxins. Although NrdH redoxins are supposed to be another candidate in the antioxidant system, their physiological roles in oxidative stress remain unclear. In this study, we confirmed that the Corynebacterium glutamicum NrdH redoxin catalytically reduces the disulfides in the class Ib ribonucleotide reductases (RNR), insulin and 5,5'-dithiobis-(2-nitrobenzoic acid) (DTNB), by exclusively receiving electrons from thioredoxin reductase. Overexpression of NrdH increased the resistance of C. glutamicum to multiple oxidative stresses by reducing ROS accumulation. Accordingly, elevated expression of the nrdH gene was observed when the C. glutamicum wild-type strain was exposed to oxidative stress conditions. It was discovered that the NrdH-mediated resistance to oxidative stresses was largely dependent on the presence of the thiol peroxidase Prx, as the increased resistance to oxidative stresses mediated by overexpression of NrdH was largely abrogated in the prx mutant. Furthermore, we showed that NrdH facilitated the hydroperoxide reduction activity of Prx by directly targeting and serving as its electron donor. Thus, we present evidence that the NrdH redoxin can protect against the damaging effects of reactive oxygen species (ROS) induced by various exogenous oxidative stresses by acting as a peroxidase cofactor.
Figures


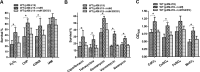

References
-
- Laurent TC, Moore EC, Reichard P. 1964. Enzymatic synthesis of deoxyribonucleotides. IV. Isolation and characterization of thioredoxin, the hydrogen donor from Escherichia coli B. J. Biol. Chem. 239:3436–3444 - PubMed
-
- Holmgren A. 1979. Glutathione-dependent synthesis of deoxyribonucleotides. Characterization of the enzymatic mechanism of Escherichia coli glutaredoxin. J. Biol. Chem. 254:3672–3678 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

